Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species.
Karakus, U., Sahin, D., Mittl, P.R.E., Mooij, P., Koopman, G., Boyman, O.(2020) Sci Transl Med 12
- PubMed: 33328333
- DOI: https://doi.org/10.1126/scitranslmed.abb9283
- Primary Citation of Related Structures:
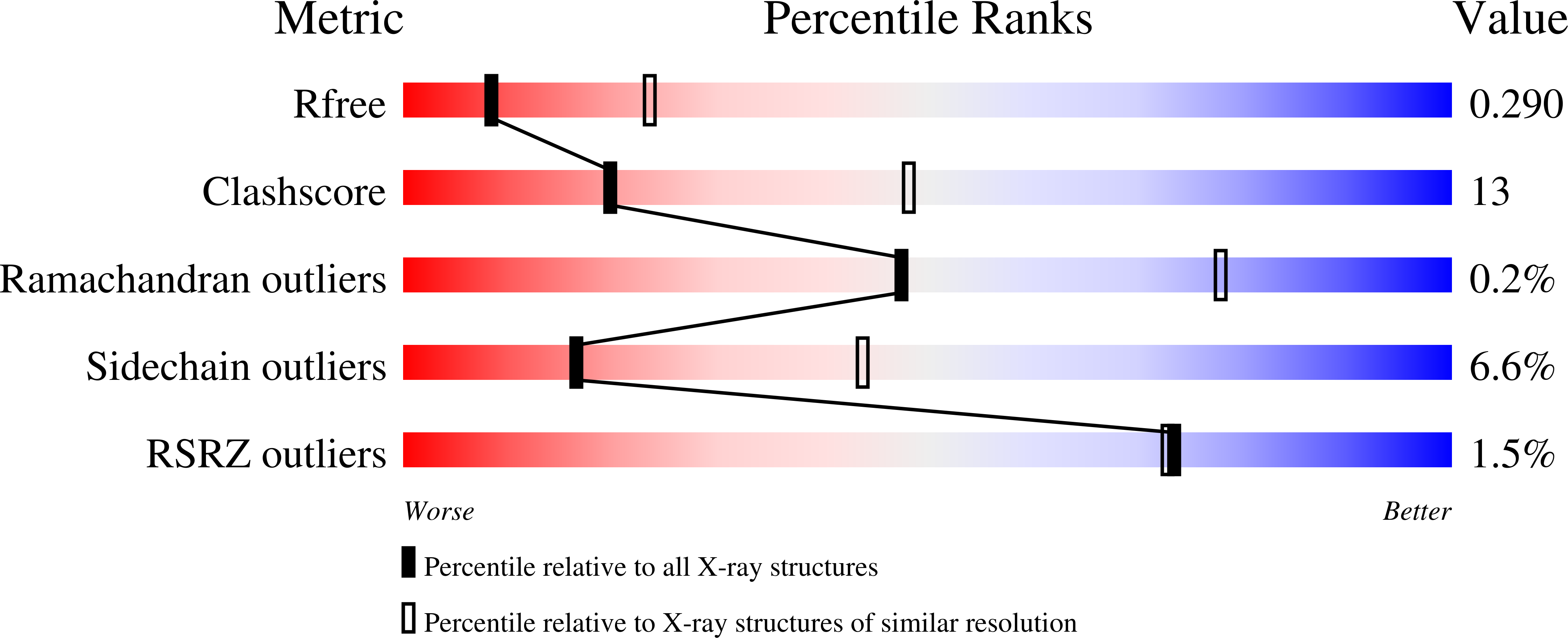
6YE3 - PubMed Abstract:
Stimulation of regulatory T (T reg ) cells holds great promise for the treatment of autoimmune, chronic inflammatory, and certain metabolic diseases. Recent clinical trials with low-dose interleukin-2 (IL-2) to expand T reg cells led to beneficial results in autoimmunity, but IL-2 immunotherapy can activate both T reg cells and pathogenic T cells. Use of IL-2 receptor α (IL-2Rα, CD25)-biased IL-2/anti-IL-2 antibody complexes improves IL-2 selectivity for T reg cells; however, the mechanism of action of such IL-2 complexes is incompletely understood, thus hampering their translation into clinical trials. Using a cell-based and dynamic IL-2R platform, we identified a particular anti-human IL-2 antibody, termed UFKA-20. When bound to UFKA-20, IL-2 failed to stimulate cells expressing IL-2Rβ (CD122) and IL-2Rγ (CD132), unless these cells also expressed high amounts of CD25. CD25 allowed IL-2/UFKA-20 complexes to bind, and binding to CD25 in the presence of CD122 and CD132 was followed by rapid dissociation of UFKA-20 from IL-2, delivery of IL-2 to CD122 and CD132, and intracellular signaling. IL-2/UFKA-20 complexes efficiently and preferentially stimulated CD4 + T reg cells in freshly isolated human T cells ex vivo and in mice and rhesus macaques in vivo. The crystal structure of the IL-2/UFKA-20 complex demonstrated that UFKA-20 interfered with IL-2 binding to CD122 and, to a lesser extent, also CD25. Together, we translated CD25-biased IL-2 complexes from mice to nonhuman primates and extended our mechanistic understanding of how CD25-biasing anti-human IL-2 antibodies work, which paves the way to clinical trials of CD25-biased IL-2 complexes.
Organizational Affiliation:
Department of Immunology, University Hospital Zurich, CH-8091 Zurich, Switzerland.