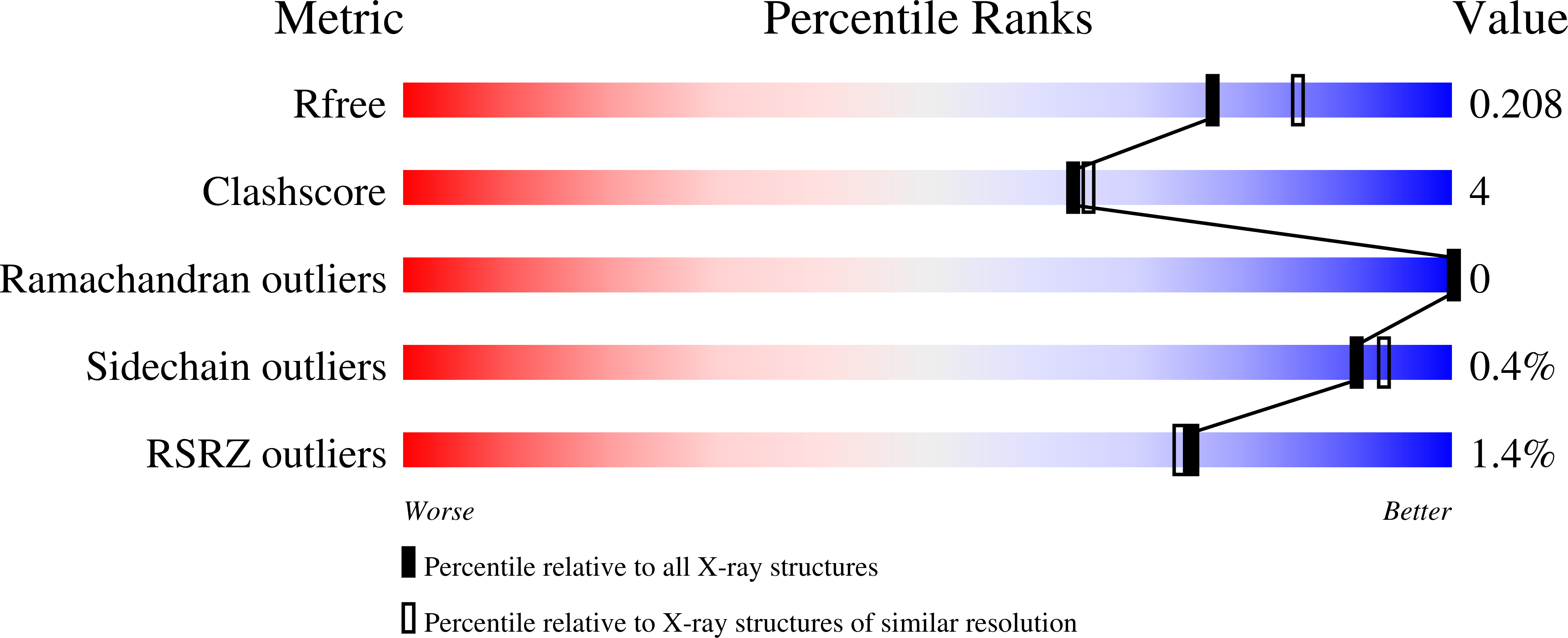
A di-iron protein recruited as an Fe[II] and oxygen sensor for bacterial chemotaxis functions by stabilizing an iron-peroxy species.
Muok, A.R., Deng, Y., Gumerov, V.M., Chong, J.E., DeRosa, J.R., Kurniyati, K., Coleman, R.E., Lancaster, K.M., Li, C., Zhulin, I.B., Crane, B.R.(2019) Proc Natl Acad Sci U S A 116: 14955-14960
- PubMed: 31270241
- DOI: https://doi.org/10.1073/pnas.1904234116
- Primary Citation of Related Structures:
6QNM, 6QRQ, 6QWO, 6R9N - PubMed Abstract:
Many bacteria contain cytoplasmic chemoreceptors that lack sensor domains. Here, we demonstrate that such cytoplasmic receptors found in 8 different bacterial and archaeal phyla genetically couple to metalloproteins related to β-lactamases and nitric oxide reductases. We show that this oxygen-binding di-iron protein (ODP) acts as a sensor for chemotactic responses to both iron and oxygen in the human pathogen Treponema denticola ( Td ). The ODP di-iron site binds oxygen at high affinity to reversibly form an unusually stable μ-peroxo adduct. Crystal structures of ODP from Td and the thermophile Thermotoga maritima ( Tm ) in the Fe[III] 2 -O 2 2- , Zn[II], and apo states display differences in subunit association, conformation, and metal coordination that indicate potential mechanisms for sensing. In reconstituted systems, iron-peroxo ODP destabilizes the phosphorylated form of the receptor-coupled histidine kinase CheA, thereby providing a biochemical link between oxygen sensing and chemotaxis in diverse prokaryotes, including anaerobes of ancient origin.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853.