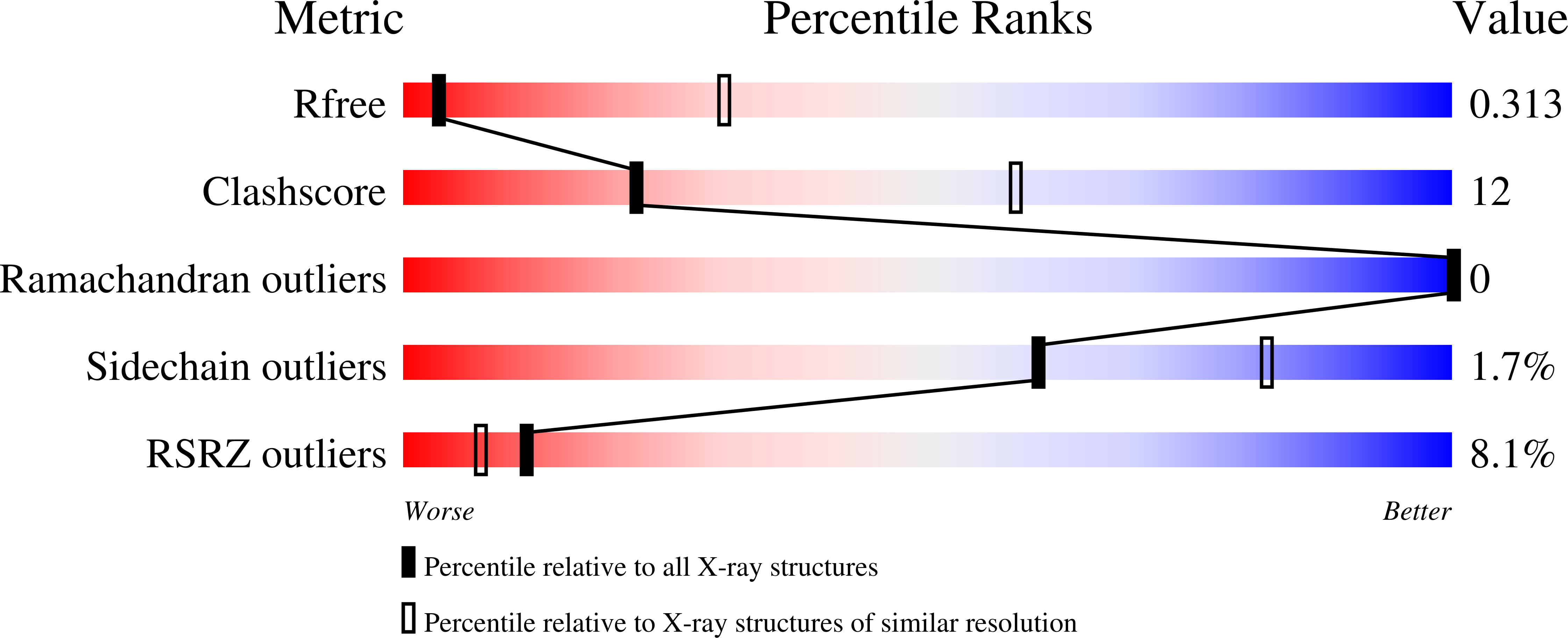
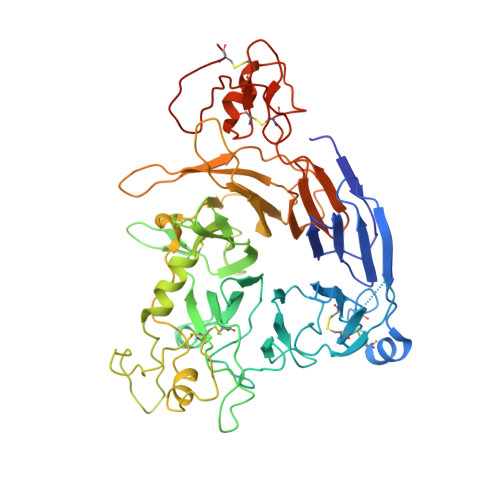
Diversity of oligomerization in Drosophila semaphorins suggests a mechanism of functional fine-tuning.
Rozbesky, D., Robinson, R.A., Jain, V., Renner, M., Malinauskas, T., Harlos, K., Siebold, C., Jones, E.Y.(2019) Nat Commun 10: 3691-3691
- PubMed: 31417095
- DOI: https://doi.org/10.1038/s41467-019-11683-y
- Primary Citation of Related Structures:
6FKK, 6QP7, 6QP8, 6QP9 - PubMed Abstract:
Semaphorin ligands and their plexin receptors are one of the major cell guidance factors that trigger localised changes in the cytoskeleton. Binding of semaphorin homodimer to plexin brings two plexins in close proximity which is a prerequisite for plexin signalling. This model appears to be too simplistic to explain the complexity and functional versatility of these molecules. Here, we determine crystal structures for all members of Drosophila class 1 and 2 semaphorins. Unlike previously reported semaphorin structures, Sema1a, Sema2a and Sema2b show stabilisation of sema domain dimer formation via a disulfide bond. Unexpectedly, our structural and biophysical data show Sema1b is a monomer suggesting that semaphorin function may not be restricted to dimers. We demonstrate that semaphorins can form heterodimers with members of the same semaphorin class. This heterodimerization provides a potential mechanism for cross-talk between different plexins and co-receptors to allow fine-tuning of cell signalling.
Organizational Affiliation:
Division of Structural Biology, Wellcome Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford, OX3 7BN, UK. daniel@strubi.ox.ac.uk.