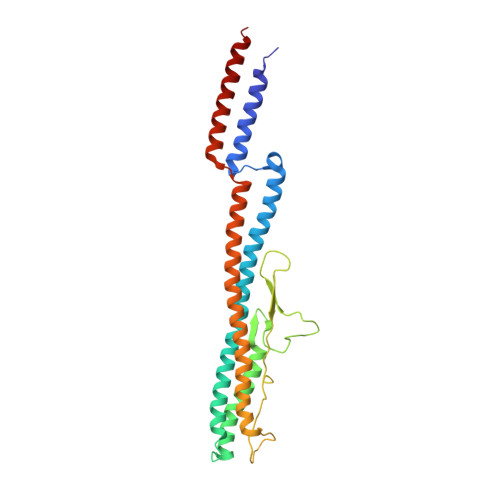
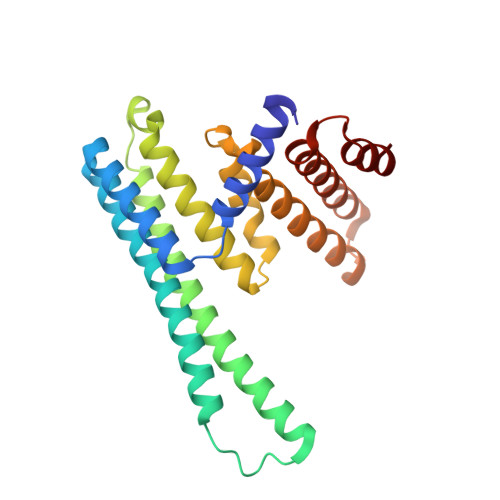
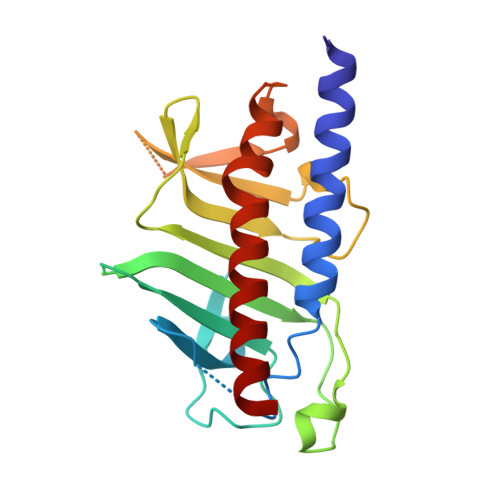
An asymmetric sheath controls flagellar supercoiling and motility in the leptospira spirochete.
Gibson, K.H., Trajtenberg, F., Wunder, E.A., Brady, M.R., San Martin, F., Mechaly, A., Shang, Z., Liu, J., Picardeau, M., Ko, A., Buschiazzo, A., Sindelar, C.V.(2020) Elife 9
- PubMed: 32157997
- DOI: https://doi.org/10.7554/eLife.53672
- Primary Citation of Related Structures:
6PWB - PubMed Abstract:
Spirochete bacteria, including important pathogens, exhibit a distinctive means of swimming via undulations of the entire cell. Motility is powered by the rotation of supercoiled 'endoflagella' that wrap around the cell body, confined within the periplasmic space. To investigate the structural basis of flagellar supercoiling, which is critical for motility, we determined the structure of native flagellar filaments from the spirochete Leptospira by integrating high-resolution cryo-electron tomography and X-ray crystallography. We show that these filaments are coated by a highly asymmetric, multi-component sheath layer, contrasting with flagellin-only homopolymers previously observed in exoflagellated bacteria. Distinct sheath proteins localize to the filament inner and outer curvatures to define the supercoiling geometry, explaining a key functional attribute of this spirochete flagellum.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale School of Medicine, New Haven, United States.