De novo protein design by citizen scientists.
Koepnick, B., Flatten, J., Husain, T., Ford, A., Silva, D.A., Bick, M.J., Bauer, A., Liu, G., Ishida, Y., Boykov, A., Estep, R.D., Kleinfelter, S., Norgard-Solano, T., Wei, L., Players, F., Montelione, G.T., DiMaio, F., Popovic, Z., Khatib, F., Cooper, S., Baker, D.(2019) Nature 570: 390-394
- PubMed: 31168091
- DOI: https://doi.org/10.1038/s41586-019-1274-4
- Primary Citation of Related Structures:
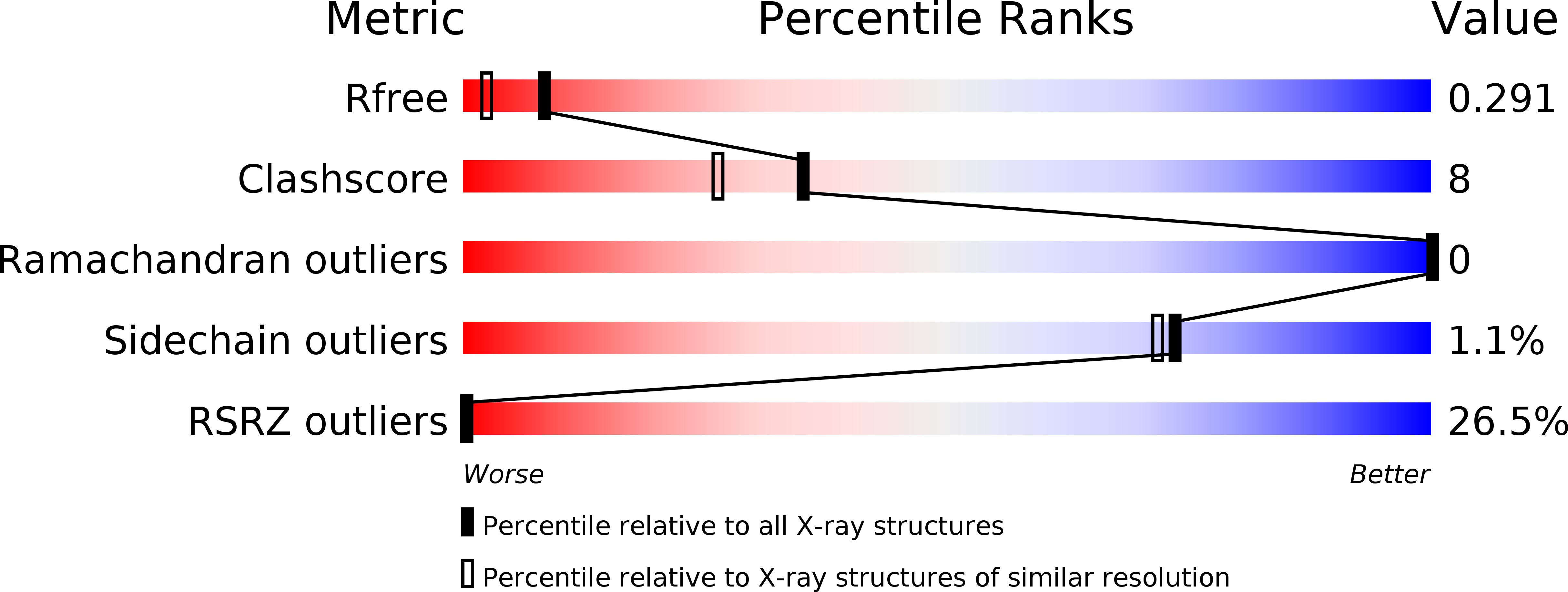
6MRR, 6MRS, 6MSP, 6NUK - PubMed Abstract:
Online citizen science projects such as GalaxyZoo 1 , Eyewire 2 and Phylo 3 have proven very successful for data collection, annotation and processing, but for the most part have harnessed human pattern-recognition skills rather than human creativity. An exception is the game EteRNA 4 , in which game players learn to build new RNA structures by exploring the discrete two-dimensional space of Watson-Crick base pairing possibilities. Building new proteins, however, is a more challenging task to present in a game, as both the representation and evaluation of a protein structure are intrinsically three-dimensional. We posed the challenge of de novo protein design in the online protein-folding game Foldit 5 . Players were presented with a fully extended peptide chain and challenged to craft a folded protein structure and an amino acid sequence encoding that structure. After many iterations of player design, analysis of the top-scoring solutions and subsequent game improvement, Foldit players can now-starting from an extended polypeptide chain-generate a diversity of protein structures and sequences that encode them in silico. One hundred forty-six Foldit player designs with sequences unrelated to naturally occurring proteins were encoded in synthetic genes; 56 were found to be expressed and soluble in Escherichia coli, and to adopt stable monomeric folded structures in solution. The diversity of these structures is unprecedented in de novo protein design, representing 20 different folds-including a new fold not observed in natural proteins. High-resolution structures were determined for four of the designs, and are nearly identical to the player models. This work makes explicit the considerable implicit knowledge that contributes to success in de novo protein design, and shows that citizen scientists can discover creative new solutions to outstanding scientific challenges such as the protein design problem.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA, USA.