Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2.
Yan, R., Zhang, Y., Li, Y., Xia, L., Guo, Y., Zhou, Q.(2020) Science 367: 1444-1448
- PubMed: 32132184
- DOI: https://doi.org/10.1126/science.abb2762
- Primary Citation of Related Structures:
6M17, 6M18, 6M1D - PubMed Abstract:
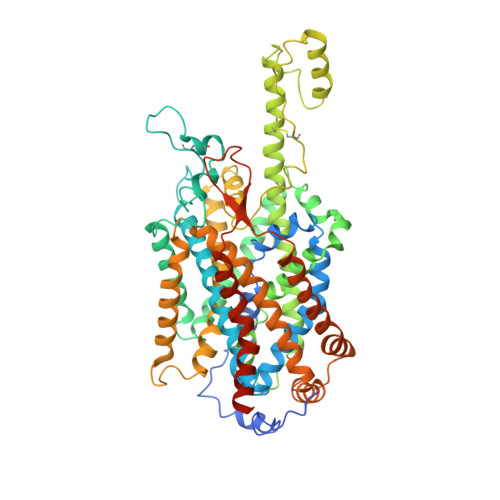
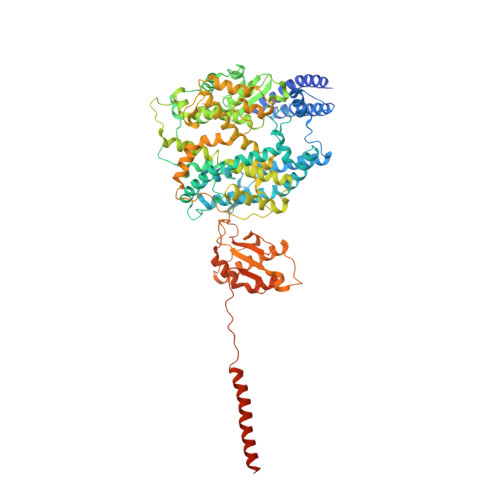
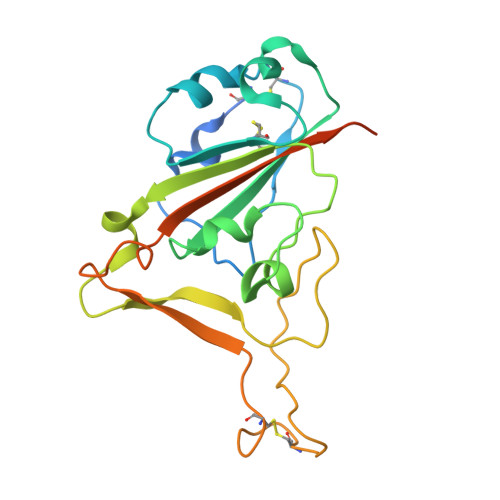
Angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for severe acute respiratory syndrome-coronavirus (SARS-CoV) and the new coronavirus (SARS-CoV-2) that is causing the serious coronavirus disease 2019 (COVID-19) epidemic. Here, we present cryo-electron microscopy structures of full-length human ACE2 in the presence of the neutral amino acid transporter B 0 AT1 with or without the receptor binding domain (RBD) of the surface spike glycoprotein (S protein) of SARS-CoV-2, both at an overall resolution of 2.9 angstroms, with a local resolution of 3.5 angstroms at the ACE2-RBD interface. The ACE2-B 0 AT1 complex is assembled as a dimer of heterodimers, with the collectrin-like domain of ACE2 mediating homodimerization. The RBD is recognized by the extracellular peptidase domain of ACE2 mainly through polar residues. These findings provide important insights into the molecular basis for coronavirus recognition and infection.
Organizational Affiliation:
Key Laboratory of Structural Biology of Zhejiang Province, Institute of Biology, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou 310024, Zhejiang Province, China.