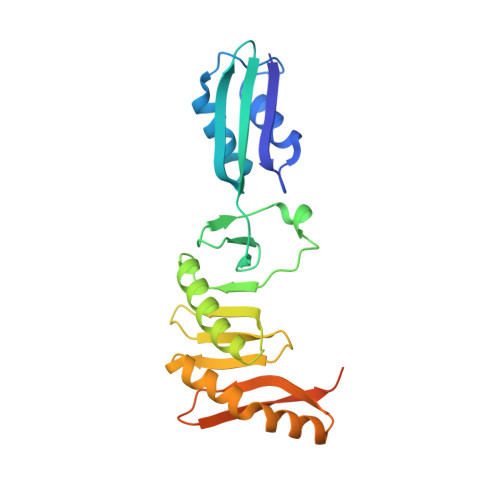
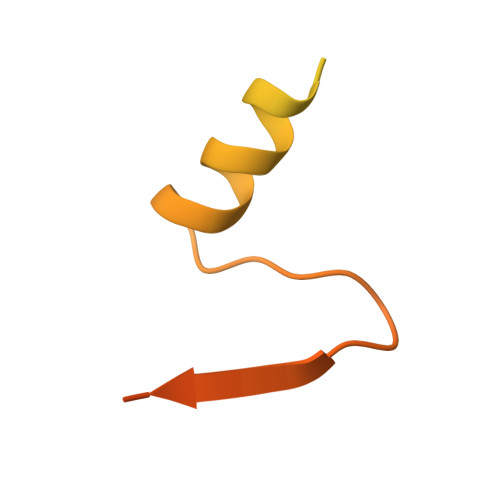
Structural Analysis of the Interaction between the Bacterial Cell Division Proteins FtsQ and FtsB.
Kureisaite-Ciziene, D., Varadajan, A., McLaughlin, S.H., Glas, M., Monton Silva, A., Luirink, R., Mueller, C., den Blaauwen, T., Grossmann, T.N., Luirink, J., Lowe, J.(2018) mBio 9
- PubMed: 30206170
- DOI: https://doi.org/10.1128/mBio.01346-18
- Primary Citation of Related Structures:
6H9N, 6H9O - PubMed Abstract:
Most bacteria and archaea use the tubulin homologue FtsZ as its central organizer of cell division. In Gram-negative Escherichia coli bacteria, FtsZ recruits cytosolic, transmembrane, periplasmic, and outer membrane proteins, assembling the divisome that facilitates bacterial cell division. One such divisome component, FtsQ, a bitopic membrane protein with a globular domain in the periplasm, has been shown to interact with many other divisome proteins. Despite its otherwise unknown function, it has been shown to be a major divisome interaction hub. Here, we investigated the interactions of FtsQ with FtsB and FtsL, two small bitopic membrane proteins that act immediately downstream of FtsQ. We show in biochemical assays that the periplasmic domains of E. coli FtsB and FtsL interact with FtsQ, but not with each other. Our crystal structure of FtsB bound to the β domain of FtsQ shows that only residues 64 to 87 of FtsB interact with FtsQ. A synthetic peptide comprising those 24 FtsB residues recapitulates the FtsQ-FtsB interactions. Protein deletions and structure-guided mutant analyses validate the structure. Furthermore, the same structure-guided mutants show cell division defects in vivo that are consistent with our structure of the FtsQ-FtsB complex that shows their interactions as they occur during cell division. Our work provides intricate details of the interactions within the divisome and also provides a tantalizing view of a highly conserved protein interaction in the periplasm of bacteria that is an excellent target for cell division inhibitor searches. IMPORTANCE In most bacteria and archaea, filaments of FtsZ protein organize cell division. FtsZ forms a ring structure at the division site and starts the recruitment of 10 to 20 downstream proteins that together form a multiprotein complex termed the divisome. The divisome is thought to facilitate many of the steps required to make two cells out of one. FtsQ and FtsB are part of the divisome, with FtsQ being a central hub, interacting with most of the other divisome components. Here we show for the first time in detail how FtsQ interacts with its downstream partner FtsB and show that mutations that disturb the interface between the two proteins effectively inhibit cell division.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, United Kingdom.