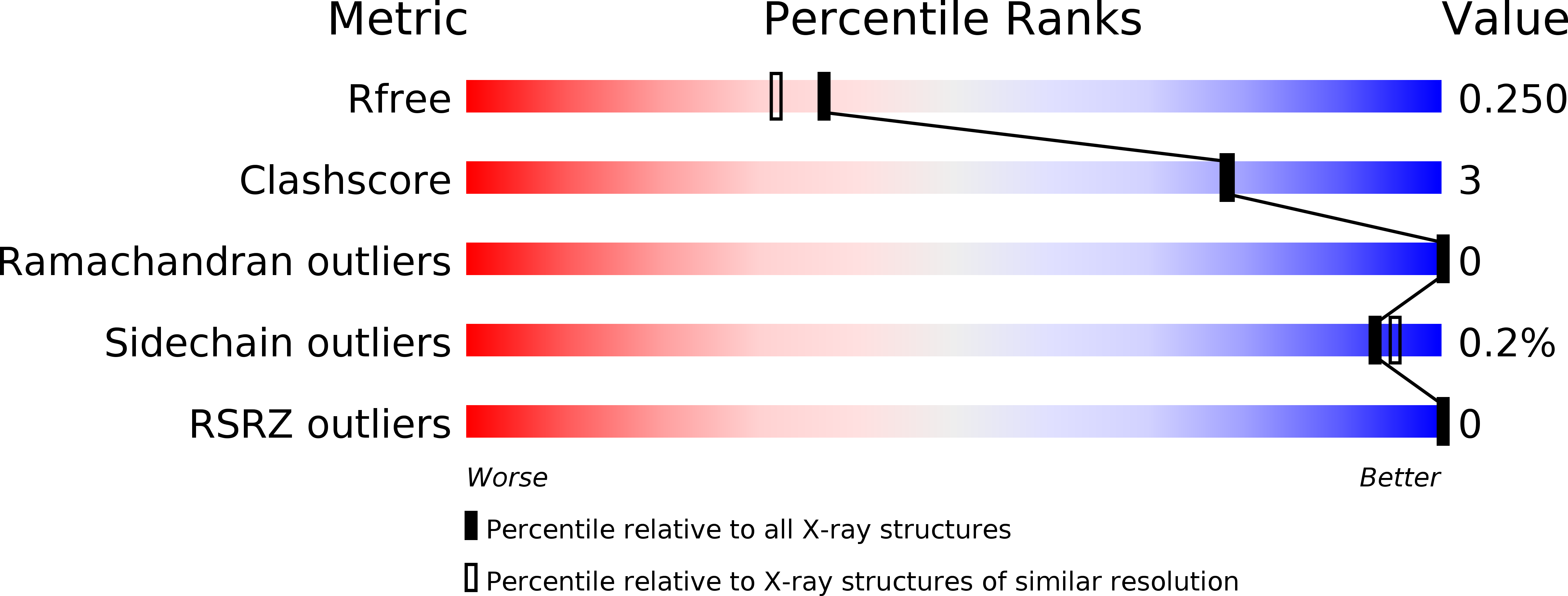
Molecular mechanism of two nanobodies that inhibit PAI-1 activity reveals a modulation at distinct stages of the PAI-1/plasminogen activator interaction.
Sillen, M., Weeks, S.D., Zhou, X., Komissarov, A.A., Florova, G., Idell, S., Strelkov, S.V., Declerck, P.J.(2020) J Thromb Haemost 18: 681-692
- PubMed: 31858714
- DOI: https://doi.org/10.1111/jth.14716
- Primary Citation of Related Structures:
6GWN, 6GWP, 6GWQ - PubMed Abstract:
Plasminogen activator inhibitor-1 (PAI-1), a key inhibitor of plasminogen activators (PAs) tissue-type PA (tPA) and urokinase-type PA (uPA) plays a crucial role in many (patho)physiological processes (e.g., cardiovascular disease, tissue fibrosis) as well as in many age-related pathologies. Therefore, much effort has been put into the development of small molecule or antibody-based PAI-1 inhibitors.
Organizational Affiliation:
Laboratory for Therapeutic and Diagnostic Antibodies, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium.