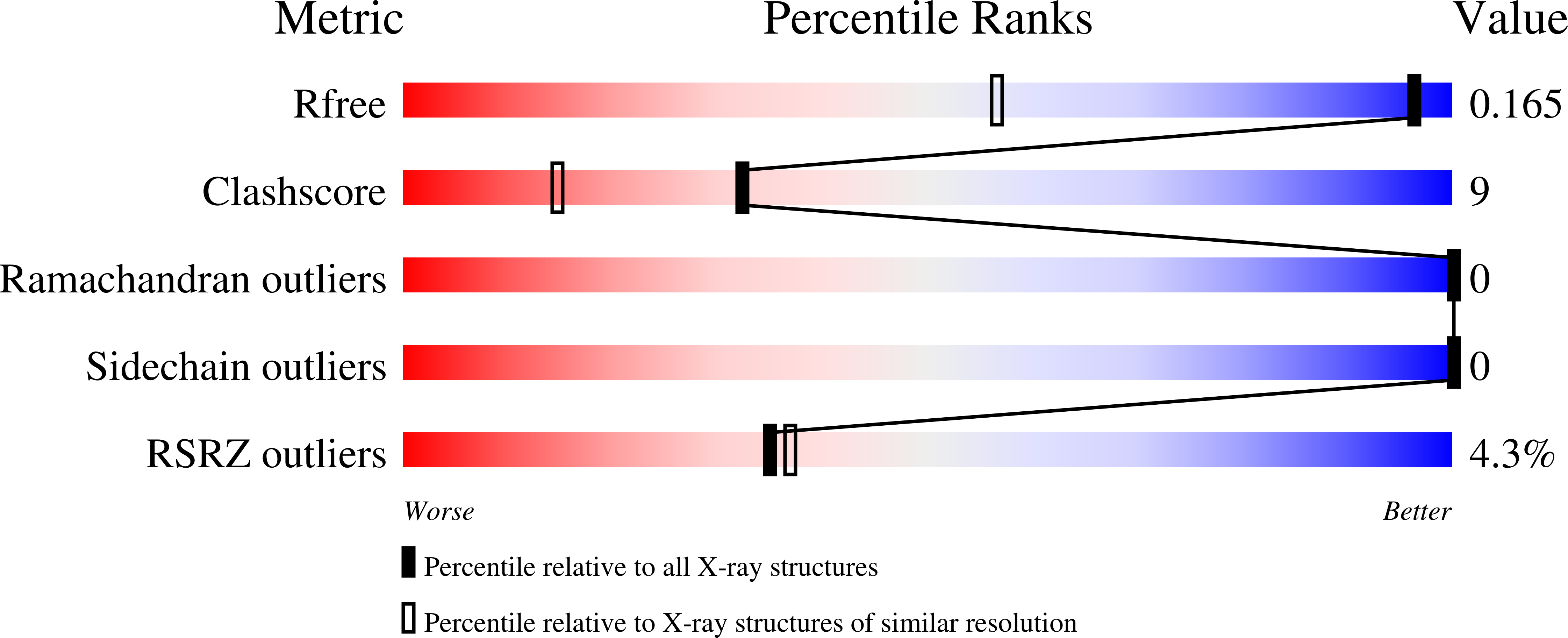
Tailing miniSOG: structural bases of the complex photophysics of a flavin-binding singlet oxygen photosensitizing protein.
Torra, J., Lafaye, C., Signor, L., Aumonier, S., Flors, C., Shu, X., Nonell, S., Gotthard, G., Royant, A.(2019) Sci Rep 9: 2428-2428
- PubMed: 30787421
- DOI: https://doi.org/10.1038/s41598-019-38955-3
- Primary Citation of Related Structures:
6GPU, 6GPV - PubMed Abstract:
miniSOG is the first flavin-binding protein that has been developed with the specific aim of serving as a genetically-encodable light-induced source of singlet oxygen ( 1 O 2 ). We have determined its 1.17 Å resolution structure, which has allowed us to investigate its mechanism of photosensitization using an integrated approach combining spectroscopic and structural methods. Our results provide a structural framework to explain the ability of miniSOG to produce 1 O 2 as a competition between oxygen- and protein quenching of its triplet state. In addition, a third excited-state decay pathway has been identified that is pivotal for the performance of miniSOG as 1 O 2 photosensitizer, namely the photo-induced transformation of flavin mononucleotide (FMN) into lumichrome, which increases the accessibility of oxygen to the flavin FMN chromophore and makes protein quenching less favourable. The combination of the two effects explains the increase in the 1 O 2 quantum yield by one order of magnitude upon exposure to blue light. Besides, we have identified several surface electron-rich residues that are progressively photo-oxidized, further contributing to facilitate the production of 1 O 2 . Our results help reconcile the apparent poor level of 1 O 2 generation by miniSOG and its excellent performance in correlative light and electron microscopy experiments.
Organizational Affiliation:
Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, Barcelona, 08017, Spain.