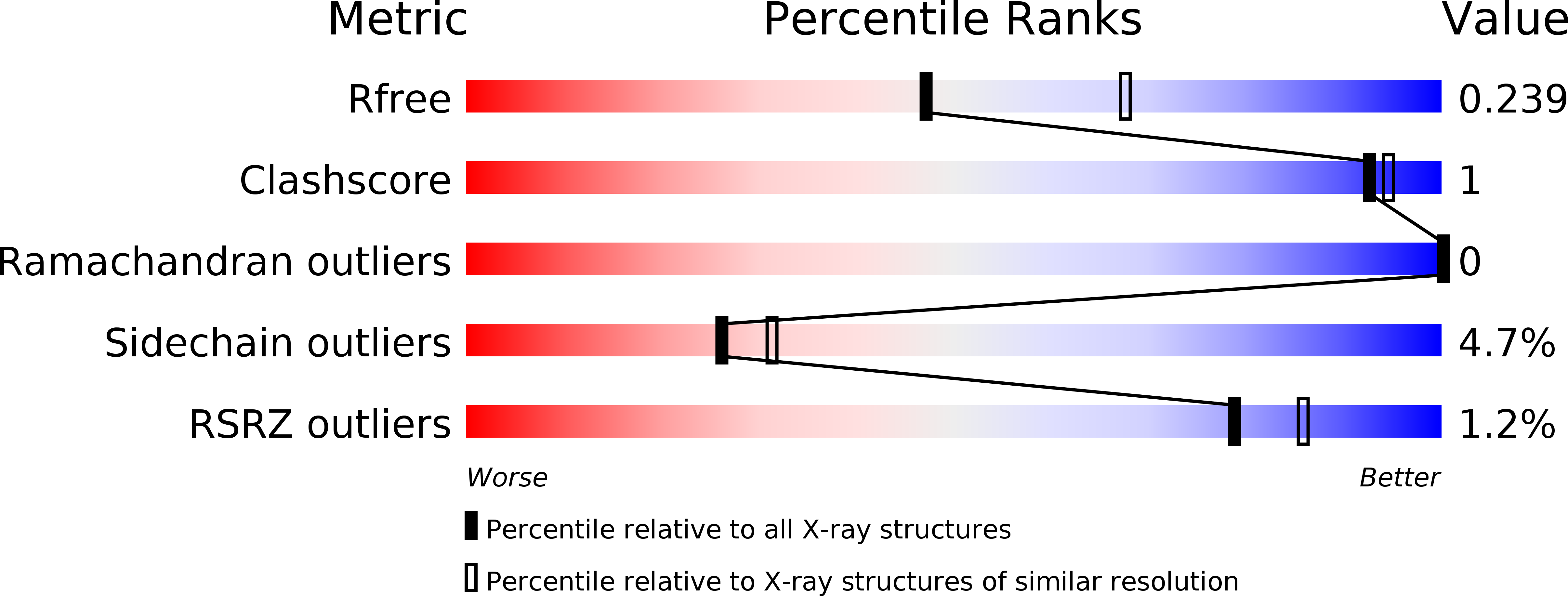
Insights into the Substrate Specificity of Archaeal Entner-Doudoroff Aldolases: The Structures of Picrophilus torridus 2-Keto-3-deoxygluconate Aldolase and Sulfolobus solfataricus 2-Keto-3-deoxy-6-phosphogluconate Aldolase in Complex with 2-Keto-3-deoxy-6-phosphogluconate.
Zaitsev, V., Johnsen, U., Reher, M., Ortjohann, M., Taylor, G.L., Danson, M.J., Schonheit, P., Crennell, S.J.(2018) Biochemistry 57: 3797-3806
- PubMed: 29812914
- DOI: https://doi.org/10.1021/acs.biochem.8b00535
- Primary Citation of Related Structures:
4UXD, 6G3Z - PubMed Abstract:
The thermoacidophilic archaea Picrophilus torridus and Sulfolobus solfataricus catabolize glucose via a nonphosphorylative Entner-Doudoroff pathway and a branched Entner-Doudoroff pathway, respectively. Key enzymes for these Entner-Doudoroff pathways are the aldolases, 2-keto-3-deoxygluconate aldolase (KDG-aldolase) and 2-keto-3-deoxy-6-phosphogluconate aldolase [KD(P)G-aldolase]. KDG-aldolase from P. torridus (Pt-KDG-aldolase) is highly specific for the nonphosphorylated substrate, 2-keto-3-deoxygluconate (KDG), whereas KD(P)G-aldolase from S. solfataricus [Ss-KD(P)G-aldolase] is an enzyme that catalyzes the cleavage of both KDG and 2-keto-3-deoxy-6-phosphogluconate (KDPG), with a preference for KDPG. The structural basis for the high specificity of Pt-KDG-aldolase for KDG as compared to the more promiscuous Ss-KD(P)G-aldolase has not been analyzed before. In this work, we report the elucidation of the structure of Ss-KD(P)G-aldolase in complex with KDPG at 2.35 Å and that of KDG-aldolase from P. torridus at 2.50 Å resolution. By superimposition of the active sites of the two enzymes, and subsequent site-directed mutagenesis studies, a network of four amino acids, namely, Arg106, Tyr132, Arg237, and Ser241, was identified in Ss-KD(P)G-aldolase that interact with the negatively charged phosphate group of KDPG, thereby increasing the affinity of the enzyme for KDPG. This KDPG-binding network is absent in Pt-KDG-aldolase, which explains the low catalytic efficiency of KDPG cleavage.
Organizational Affiliation:
Biomolecular Sciences , University of St Andrews , St Andrews , Fife KY16 9ST , U.K.