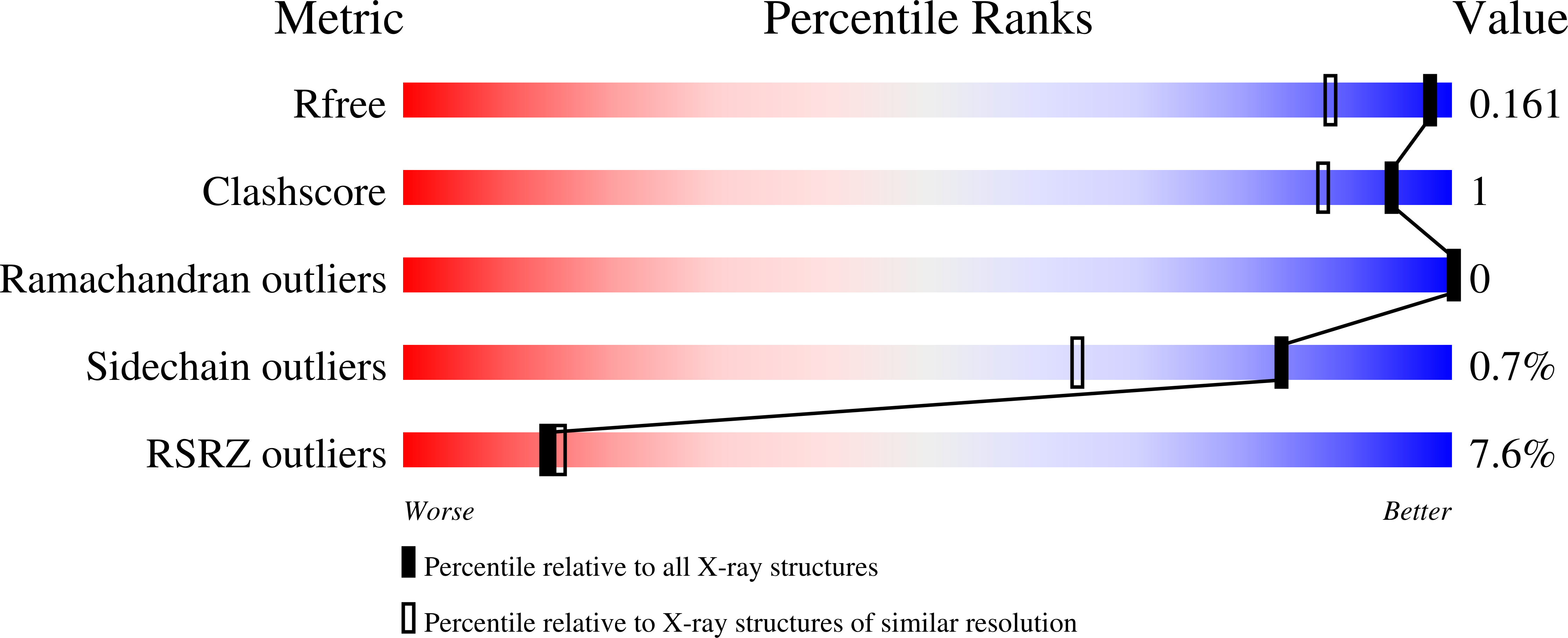
A Gcn5-Related N-Acetyltransferase (GNAT) Capable of Acetylating Polymyxin B and Colistin Antibiotics in Vitro.
Czub, M.P., Zhang, B., Chiarelli, M.P., Majorek, K.A., Joe, L., Porebski, P.J., Revilla, A., Wu, W., Becker, D.P., Minor, W., Kuhn, M.L.(2018) Biochemistry 57: 7011-7020
- PubMed: 30499668
- DOI: https://doi.org/10.1021/acs.biochem.8b00946
- Primary Citation of Related Structures:
6EDD, 6EDV - PubMed Abstract:
Deeper exploration of uncharacterized Gcn5-related N-acetyltransferases has the potential to expand our knowledge of the types of molecules that can be acylated by this important superfamily of enzymes and may offer new opportunities for biotechnological applications. While determining native or biologically relevant in vivo functions of uncharacterized proteins is ideal, their alternative or promiscuous in vitro capabilities provide insight into key active site interactions. Additionally, this knowledge can be exploited to selectively modify complex molecules and reduce byproducts when synthetic routes become challenging. During our exploration of uncharacterized Gcn5-related N-acetyltransferases from Pseudomonas aeruginosa, we identified such an example. We found that the PA3944 enzyme acetylates both polymyxin B and colistin on a single diaminobutyric acid residue closest to the macrocyclic ring of the antimicrobial peptide and determined the PA3944 crystal structure. This finding is important for several reasons. (1) To the best of our knowledge, this is the first report of enzymatic acylation of polymyxins and thus reveals a new type of substrate that this enzyme family can use. (2) The enzymatic acetylation offers a controlled method for antibiotic modification compared to classical promiscuous chemical methods. (3) The site of acetylation would reduce the overall positive charge of the molecule, which is important for reducing nephrotoxic effects and may be a salvage strategy for this important class of antibiotics. While the physiological substrate for this enzyme remains unknown, our structural and functional characterization of PA3944 offers insight into its unique noncanonical substrate specificity.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics , University of Virginia , Charlottesville , Virginia 22908 , United States.