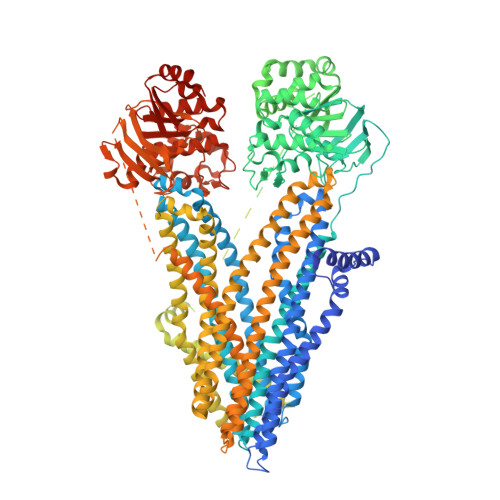
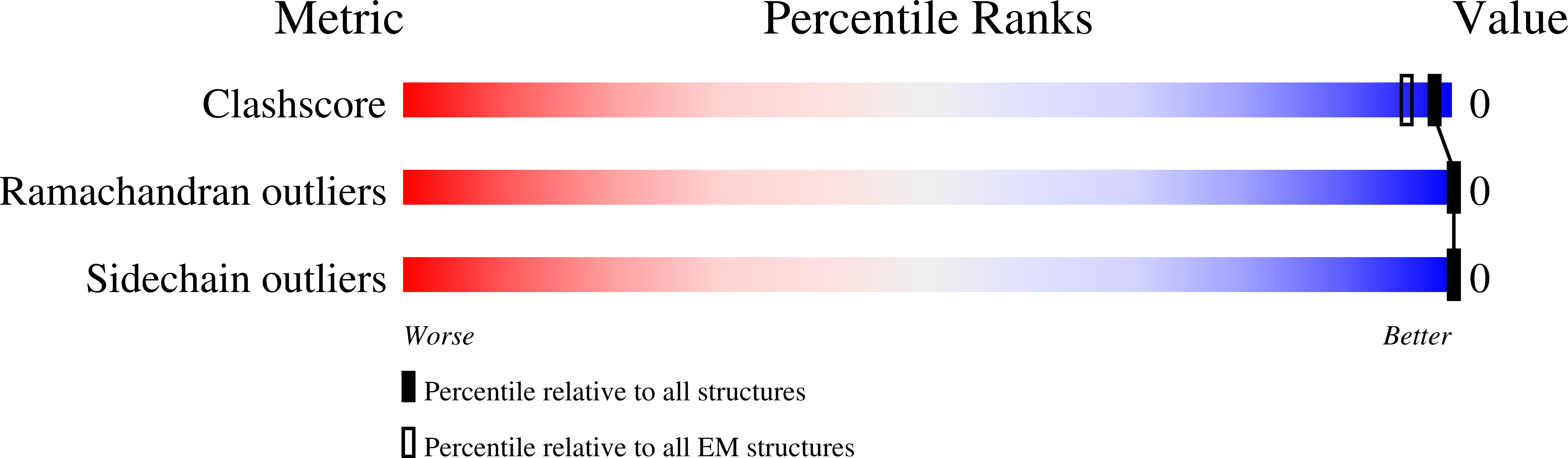Cryo-EM Visualization of an Active High Open Probability CFTR Anion Channel.
Fay, J.F., Aleksandrov, L.A., Jensen, T.J., Cui, L.L., Kousouros, J.N., He, L., Aleksandrov, A.A., Gingerich, D.S., Riordan, J.R., Chen, J.Z.(2018) Biochemistry 57: 6234-6246
- PubMed: 30281975
- DOI: https://doi.org/10.1021/acs.biochem.8b00763
- Primary Citation of Related Structures:
6D3R, 6D3S - PubMed Abstract:
The cystic fibrosis transmembrane conductance regulator (CFTR) anion channel, crucial to epithelial salt and water homeostasis, and defective due to mutations in its gene in patients with cystic fibrosis, is a unique member of the large family of ATP-binding cassette transport proteins. Regulation of CFTR channel activity is stringently controlled by phosphorylation and nucleotide binding. Structural changes that underlie transitions between active and inactive functional states are not yet fully understood. Indeed the first 3D structures of dephosphorylated, ATP-free, and phosphorylated ATP-bound states were only recently reported. Here we have determined the structure of inactive and active states of a thermally stabilized CFTR, the latter with a very high channel open probability, confirmed after reconstitution into proteoliposomes. These structures, obtained at nominal resolution of 4.3 and 6.6 Å, reveal a unique repositioning of the transmembrane helices and regulatory domain density that provide insights into the structural transition between active and inactive functional states of CFTR. Moreover, we observe an extracellular vestibule that may provide anion access to the pore due to the conformation of transmembrane helices 7 and 8 that differs from the previous orthologue CFTR structures. In conclusion, our work contributes detailed structural information on an active, open state of the CFTR anion channel.
Organizational Affiliation:
University of North Carolina , Chapel Hill , North Carolina 27515 , United States.