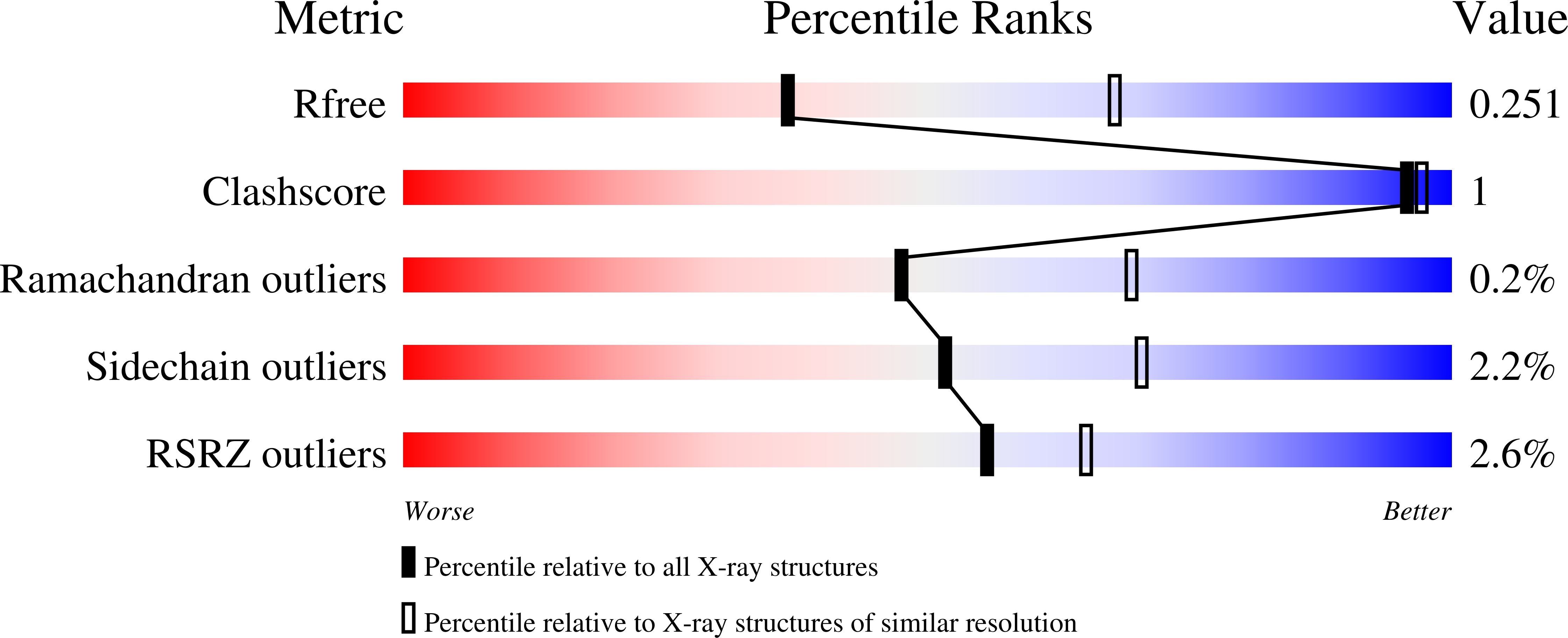
Structural and biochemical analysis of Bacillus anthracis prephenate dehydrogenase reveals an unusual mode of inhibition by tyrosine via the ACT domain.
Shabalin, I.G., Gritsunov, A., Hou, J., Slawek, J., Miks, C.D., Cooper, D.R., Minor, W., Christendat, D.(2020) FEBS J 287: 2235-2255
- PubMed: 31750992
- DOI: https://doi.org/10.1111/febs.15150
- Primary Citation of Related Structures:
5UYY, 5V0S, 6CXD, 6U60 - PubMed Abstract:
Tyrosine biosynthesis via the shikimate pathway is absent in humans and other animals, making it an attractive target for next-generation antibiotics, which is increasingly important due to the looming proliferation of multidrug-resistant pathogens. Tyrosine biosynthesis is also of commercial importance for the environmentally friendly production of numerous compounds, such as pharmaceuticals, opioids, aromatic polymers, and petrochemical aromatics. Prephenate dehydrogenase (PDH) catalyzes the penultimate step of tyrosine biosynthesis in bacteria: the oxidative decarboxylation of prephenate to 4-hydroxyphenylpyruvate. The majority of PDHs are competitively inhibited by tyrosine and consist of a nucleotide-binding domain and a dimerization domain. Certain PDHs, including several from pathogens on the World Health Organization priority list of antibiotic-resistant bacteria, possess an additional ACT domain. However, biochemical and structural knowledge was lacking for these enzymes. In this study, we successfully established a recombinant protein expression system for PDH from Bacillus anthracis (BaPDH), the causative agent of anthrax, and determined the structure of a BaPDH ternary complex with NAD + and tyrosine, a binary complex with tyrosine, and a structure of an isolated ACT domain dimer. We also conducted detailed kinetic and biophysical analyses of the enzyme. We show that BaPDH is allosterically regulated by tyrosine binding to the ACT domains, resulting in an asymmetric conformation of the BaDPH dimer that sterically prevents prephenate binding to either active site. The presented mode of allosteric inhibition is unique compared to both the competitive inhibition established for other PDHs and to the allosteric mechanisms for other ACT-containing enzymes. This study provides new structural and mechanistic insights that advance our understanding of tyrosine biosynthesis in bacteria. ENZYMES: Prephenate dehydrogenase from Bacillus anthracis (PDH): EC database ID: 1.3.1.12. DATABASES: Coordinates and structure factors have been deposited in the Protein Data Bank (PDB) with accession numbers PDB ID: 6U60 (BaPDH complex with NAD + and tyrosine), PDB ID: 5UYY (BaPDH complex with tyrosine), and PDB ID: 5V0S (BaPDH isolated ACT domain dimer). The diffraction images are available at http://proteindiffraction.org with DOIs: https://doi.org/10.18430/M35USC, https://doi.org/10.18430/M35UYY, and https://doi.org/10.18430/M35V0S.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA, USA.