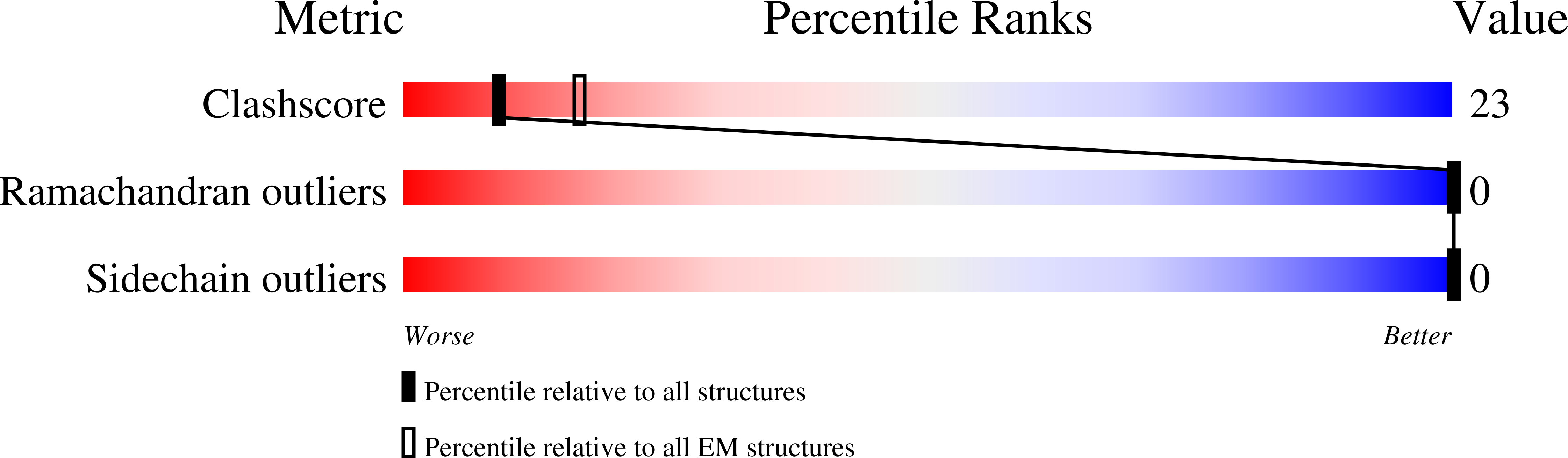Hepatitis B virus core protein allosteric modulators can distort and disrupt intact capsids.
Schlicksup, C.J., Wang, J.C., Francis, S., Venkatakrishnan, B., Turner, W.W., VanNieuwenhze, M., Zlotnick, A.(2018) Elife 7
- PubMed: 29377794
- DOI: https://doi.org/10.7554/eLife.31473
- Primary Citation of Related Structures:
6BVF, 6BVN - PubMed Abstract:
Defining mechanisms of direct-acting antivirals facilitates drug development and our understanding of virus function. Heteroaryldihydropyrimidines (HAPs) inappropriately activate assembly of hepatitis B virus (HBV) core protein (Cp), suppressing formation of virions. We examined a fluorophore-labeled HAP, HAP-TAMRA. HAP-TAMRA induced Cp assembly and also bound pre-assembled capsids. Kinetic and spectroscopic studies imply that HAP-binding sites are usually not available but are bound cooperatively. Using cryo-EM, we observed that HAP-TAMRA asymmetrically deformed capsids, creating a heterogeneous array of sharp angles, flat regions, and outright breaks. To achieve high resolution reconstruction (<4 Å), we introduced a disulfide crosslink that rescued particle symmetry. We deduced that HAP-TAMRA caused quasi-sixfold vertices to become flatter and fivefold more angular. This transition led to asymmetric faceting. That a disordered crosslink could rescue symmetry implies that capsids have tensegrity properties. Capsid distortion and disruption is a new mechanism by which molecules like the HAPs can block HBV infection.
Organizational Affiliation:
Department of Molecular and Cellular Biochemistry, Indiana University, Bloomington, United States.