Stability of cytochromes c' from psychrophilic and piezophilic Shewanella species: implications for complex multiple adaptation to low temperature and high hydrostatic pressure.
Suka, A., Oki, H., Kato, Y., Kawahara, K., Ohkubo, T., Maruno, T., Kobayashi, Y., Fujii, S., Wakai, S., Lisdiana, L., Sambongi, Y.(2019) Extremophiles 23: 239-248
- PubMed: 30689055
- DOI: https://doi.org/10.1007/s00792-019-01077-9
- Primary Citation of Related Structures:
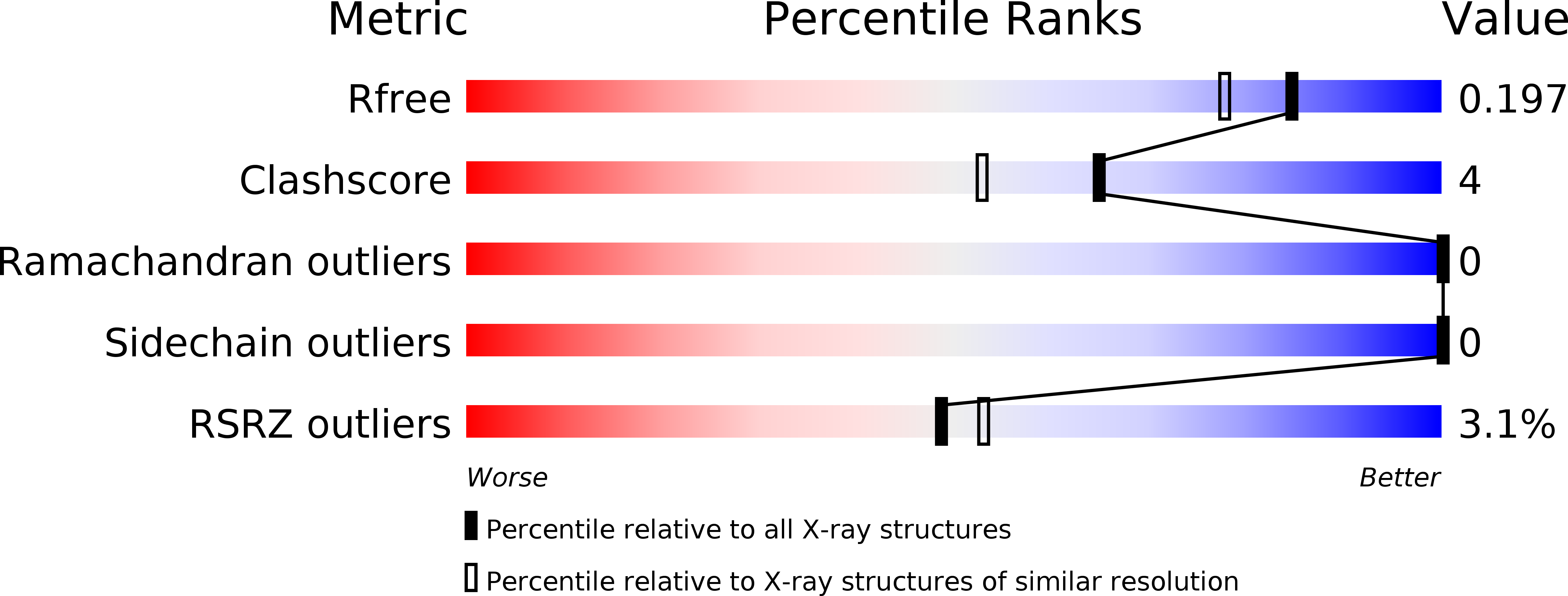
6A3K, 6A3L - PubMed Abstract:
The stability of dimeric cytochrome c' from a thermophile, as compared with that of a homologous mesophilic counterpart, is attributed to strengthened interactions around the heme and at the subunit-subunit interface, both of which are molecular interior regions. Here, we showed that interactions in the equivalent interior regions of homologous cytochromes c' from two psychrophiles, Shewanella benthica and Shewanella violacea (SBCP and SVCP, respectively) were similarly weakened as compared with those of the counterparts of psychrophilic Shewanella livingstonensis and mesophilic Shewanella amazonensis (SLCP and SACP, respectively), and consistently the stability of SVCP, SLCP, and SACP increased in that order. Therefore, the stability of cytochromes c' from the psychrophile, mesophile, and thermophile is systematically regulated in their molecular interior regions. Unexpectedly, however, the stability of SBCP was significantly higher than that of SVCP, and the former had additional molecular surface interactions. Collectively, SBCP had weakened interior interactions like SVCP did, but the former was stabilized at the molecular surface as compared with the latter, implying complex multiple adaptation of the proteins because the psychrophilic sources of SBCP and SVCP are also piezophilic, thriving in deep-sea extreme environments of low temperature and high hydrostatic pressure.
Organizational Affiliation:
Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima, 1-4-4 Kagamiyama, Hiroshima, 739-8528, Japan.