Crystal structure and functional analysis of human C1ORF123.
A Rahaman, S.N., Mat Yusop, J., Mohamed-Hussein, Z.A., Aizat, W.M., Ho, K.L., Teh, A.H., Waterman, J., Tan, B.K., Tan, H.L., Li, A.Y., Chen, E.S., Ng, C.L.(2018) PeerJ 6: e5377-e5377
- PubMed: 30280012
- DOI: https://doi.org/10.7717/peerj.5377
- Primary Citation of Related Structures:
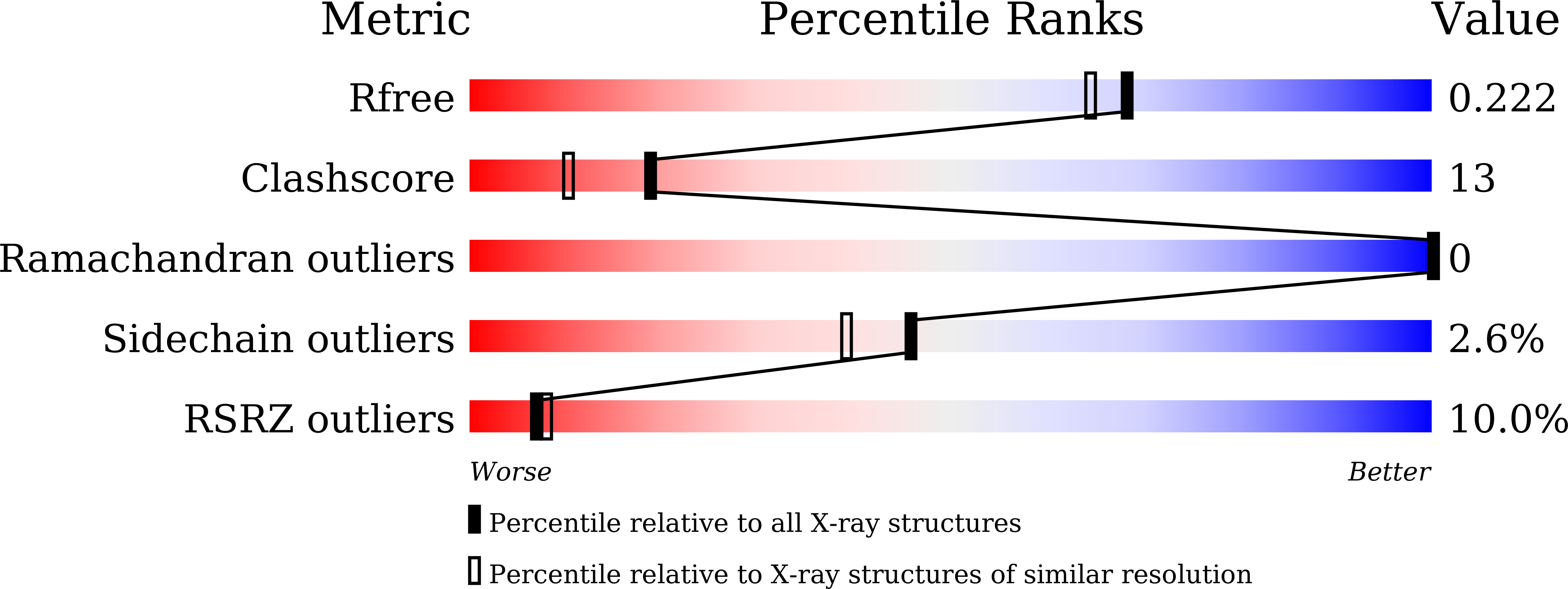
5ZRT - PubMed Abstract:
Proteins of the DUF866 superfamily are exclusively found in eukaryotic cells. A member of the DUF866 superfamily, C1ORF123, is a human protein found in the open reading frame 123 of chromosome 1. The physiological role of C1ORF123 is yet to be determined. The only available protein structure of the DUF866 family shares just 26% sequence similarity and does not contain a zinc binding motif. Here, we present the crystal structure of the recombinant human C1ORF123 protein (rC1ORF123). The structure has a 2-fold internal symmetry dividing the monomeric protein into two mirrored halves that comprise of distinct electrostatic potential. The N-terminal half of rC1ORF123 includes a zinc-binding domain interacting with a zinc ion near to a potential ligand binding cavity. Functional studies of human C1ORF123 and its homologue in the fission yeast Schizosaccharomyces pombe (SpEss1) point to a role of DUF866 protein in mitochondrial oxidative phosphorylation.
Organizational Affiliation:
Institute of Systems Biology, Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia.