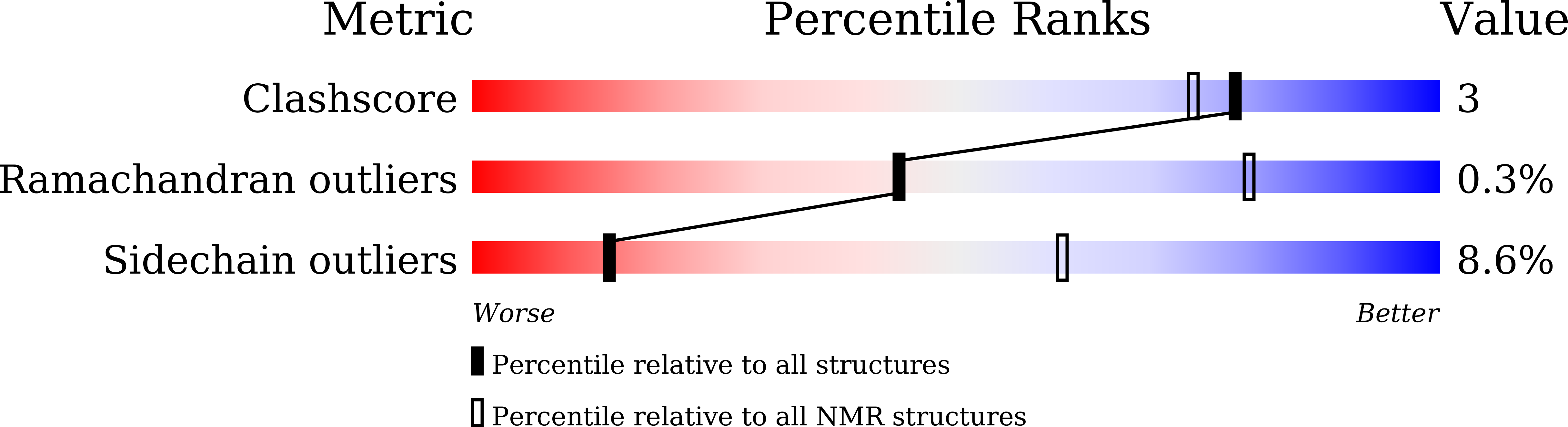NMR structure and localization of a large fragment of the SARS-CoV fusion protein: Implications in viral cell fusion.
Mahajan, M., Chatterjee, D., Bhuvaneswari, K., Pillay, S., Bhattacharjya, S.(2017) Biochim Biophys Acta 1860: 407-415
- PubMed: 28988778
- DOI: https://doi.org/10.1016/j.bbamem.2017.10.002
- Primary Citation of Related Structures:
5XJK - PubMed Abstract:
The lethal Coronaviruses (CoVs), Severe Acute Respiratory Syndrome-associated Coronavirus (SARS-CoV) and most recently Middle East Respiratory Syndrome Coronavirus, (MERS-CoV) are serious human health hazard. A successful viral infection requires fusion between virus and host cells carried out by the surface spike glycoprotein or S protein of CoV. Current models propose that the S2 subunit of S protein assembled into a hexameric helical bundle exposing hydrophobic fusogenic peptides or fusion peptides (FPs) for membrane insertion. The N-terminus of S2 subunit of SARS-CoV reported to be active in cell fusion whereby FPs have been identified. Atomic-resolution structure of FPs derived either in model membranes or in membrane mimic environment would glean insights toward viral cell fusion mechanism. Here, we have solved 3D structure, dynamics and micelle localization of a 64-residue long fusion peptide or LFP in DPC detergent micelles by NMR methods. Micelle bound structure of LFP is elucidated by the presence of discretely folded helical and intervening loops. The C-terminus region, residues F42-Y62, displays a long hydrophobic helix, whereas the N-terminus is defined by a short amphipathic helix, residues R4-Q12. The intervening residues of LFP assume stretches of loops and helical turns. The N-terminal helix is sustained by close aromatic and aliphatic sidechain packing interactions at the non-polar face. 15 N{ 1 H}NOE studies indicated dynamical motion, at ps-ns timescale, of the helices of LFP in DPC micelles. PRE NMR showed that insertion of several regions of LFP into DPC micelle core. Together, the current study provides insights toward fusion mechanism of SARS-CoV.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore.