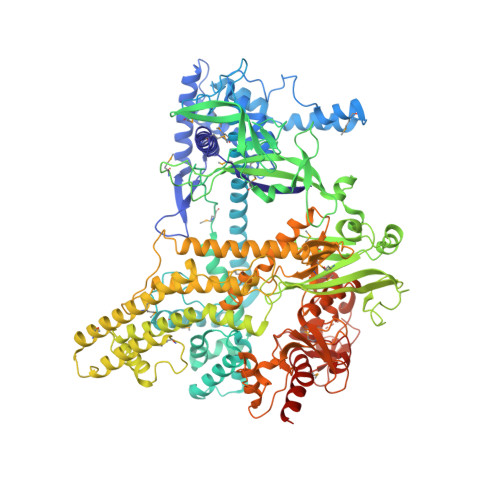
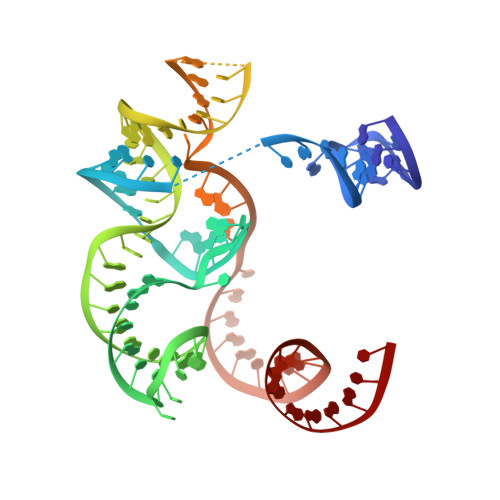
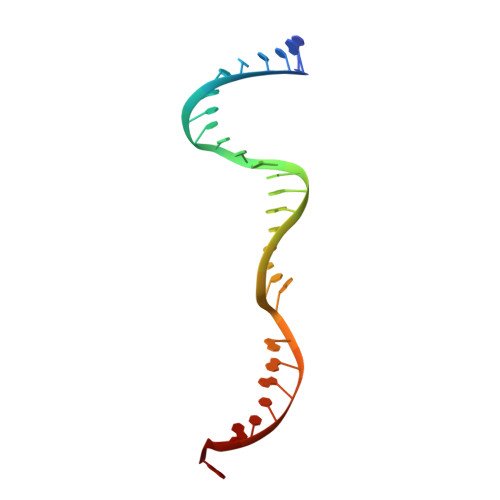
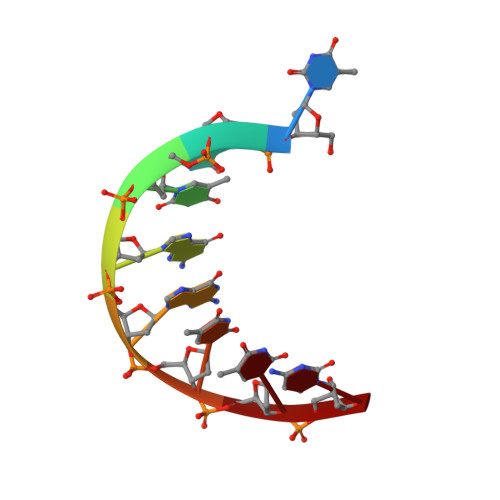
PAM-Dependent Target DNA Recognition and Cleavage by C2c1 CRISPR-Cas Endonuclease.
Yang, H., Gao, P., Rajashankar, K.R., Patel, D.J.(2016) Cell 167: 1814-1828.e12
- PubMed: 27984729
- DOI: https://doi.org/10.1016/j.cell.2016.11.053
- Primary Citation of Related Structures:
5U30, 5U31, 5U33, 5U34 - PubMed Abstract:
C2c1 is a newly identified guide RNA-mediated type V-B CRISPR-Cas endonuclease that site-specifically targets and cleaves both strands of target DNA. We have determined crystal structures of Alicyclobacillus acidoterrestris C2c1 (AacC2c1) bound to sgRNA as a binary complex and to target DNAs as ternary complexes, thereby capturing catalytically competent conformations of AacC2c1 with both target and non-target DNA strands independently positioned within a single RuvC catalytic pocket. Moreover, C2c1-mediated cleavage results in a staggered seven-nucleotide break of target DNA. crRNA adopts a pre-ordered five-nucleotide A-form seed sequence in the binary complex, with release of an inserted tryptophan, facilitating zippering up of 20-bp guide RNA:target DNA heteroduplex on ternary complex formation. Notably, the PAM-interacting cleft adopts a "locked" conformation on ternary complex formation. Structural comparison of C2c1 ternary complexes with their Cas9 and Cpf1 counterparts highlights the diverse mechanisms adopted by these distinct CRISPR-Cas systems, thereby broadening and enhancing their applicability as genome editing tools.
Organizational Affiliation:
Structurel Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Electronic address: yangh3@mskcc.org.