Target Elucidation by Cocrystal Structures of NADH-Ubiquinone Oxidoreductase of Plasmodium falciparum (PfNDH2) with Small Molecule To Eliminate Drug-Resistant Malaria
Yang, Y., Yu, Y., Li, X., Li, J., Wu, Y., Yu, J., Ge, J., Huang, Z., Jiang, L., Rao, Y., Yang, M.(2017) J Med Chem 60: 1994-2005
- PubMed: 28195463
- DOI: https://doi.org/10.1021/acs.jmedchem.6b01733
- Primary Citation of Related Structures:
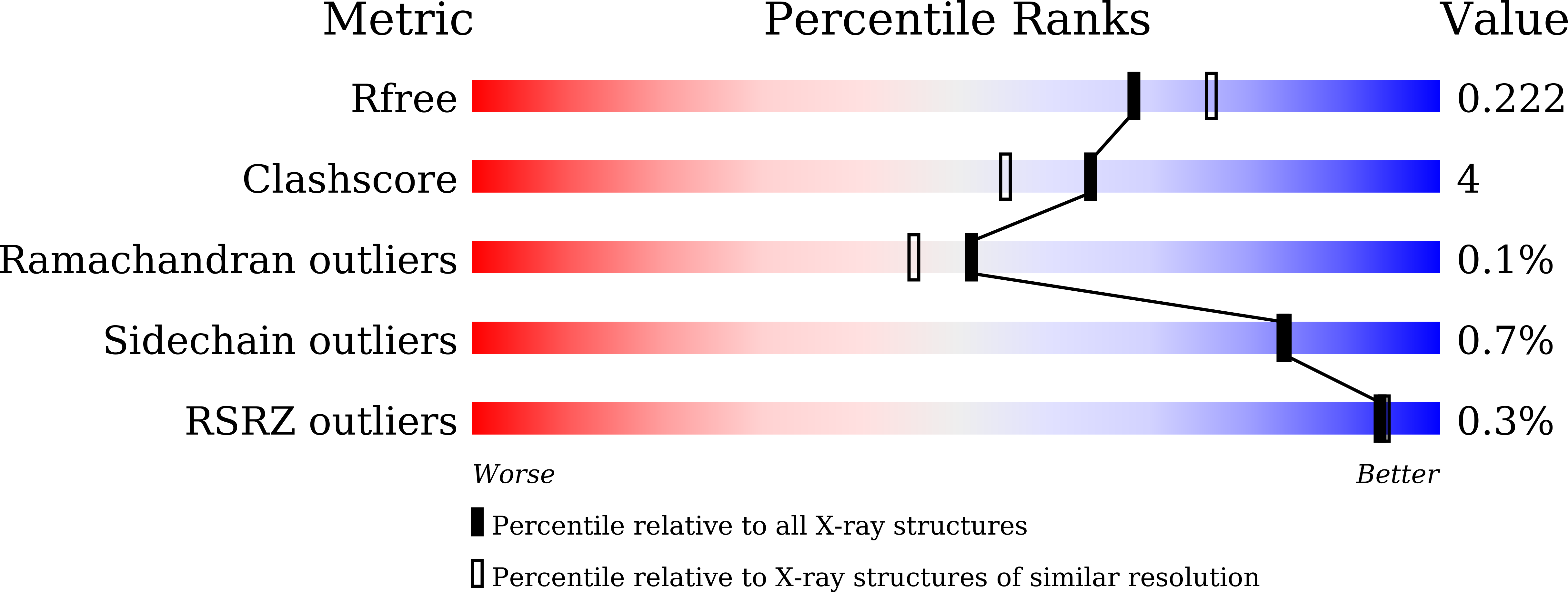
5JWA, 5JWB, 5JWC - PubMed Abstract:
Drug-resistant malarial strains have been continuously emerging recently, which posts a great challenge for the global health. Therefore, new antimalarial drugs with novel targeting mechanisms are urgently needed for fighting drug-resistant malaria. NADH-ubiquinone oxidoreductase of Plasmodium falciparum (PfNDH2) represents a viable target for antimalarial drug development. However, the absence of structural information on PfNDH2 limited rational drug design and further development. Herein, we report high resolution crystal structures of the PfNDH2 protein for the first time in Apo-, NADH-, and RYL-552 (a new inhibitor)-bound states. The PfNDH2 inhibitor exhibits excellent potency against both drug-resistant strains in vitro and parasite-infected mice in vivo via a potential allosteric mechanism. Furthermore, it was found that the inhibitor can be used in combination with dihydroartemisinin (DHA) synergistically. These findings not only are important for malarial PfNDH2 protein-based drug development but could also have broad implications for other NDH2-containing pathogenic microorganisms such as Mycobacterium tuberculosis.
Organizational Affiliation:
MOE Key Laboratory of Protein Sciences, School of Pharmaceutical Sciences, Tsinghua University , Beijing 100084, China.