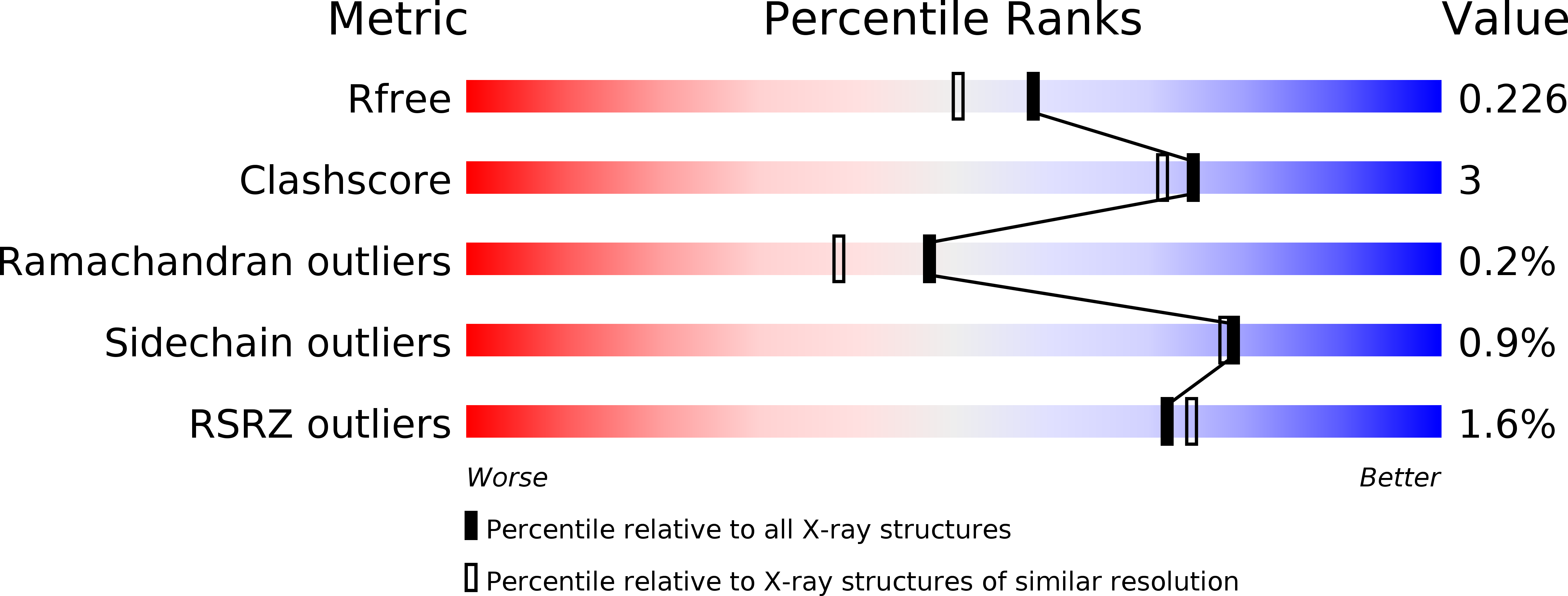
X-ray structure of linalool dehydratase/isomerase from Castellaniella defragrans reveals enzymatic alkene synthesis.
Weidenweber, S., Marmulla, R., Ermler, U., Harder, J.(2016) FEBS Lett 590: 1375-1383
- PubMed: 27062179
- DOI: https://doi.org/10.1002/1873-3468.12165
- Primary Citation of Related Structures:
5HLR, 5HSS - PubMed Abstract:
Linalool dehydratase/isomerase (Ldi), an enzyme of terpene degradation in Castellaniella defragrans, isomerizes the primary monoterpene alcohol geraniol into the tertiary alcohol (S)-linalool and dehydrates (S)-linalool to the alkene β-myrcene. Here we report on the crystal structures of Ldi with and without terpene substrates, revealing a cofactor-free homopentameric enzyme. The substrates were embedded inside a hydrophobic channel between two monomers of the (α,α)6 barrel fold class and flanked by three clusters of polar residues involved in acid-base catalysis. The detailed view into the active site will guide future biotechnological applications of Ldi, in particular, for industrial butadiene and isoprene production from renewable sources.
Organizational Affiliation:
Department of Molecular Membrane Biology, Max Planck Institute of Biophysics, Frankfurt am Main, Germany.