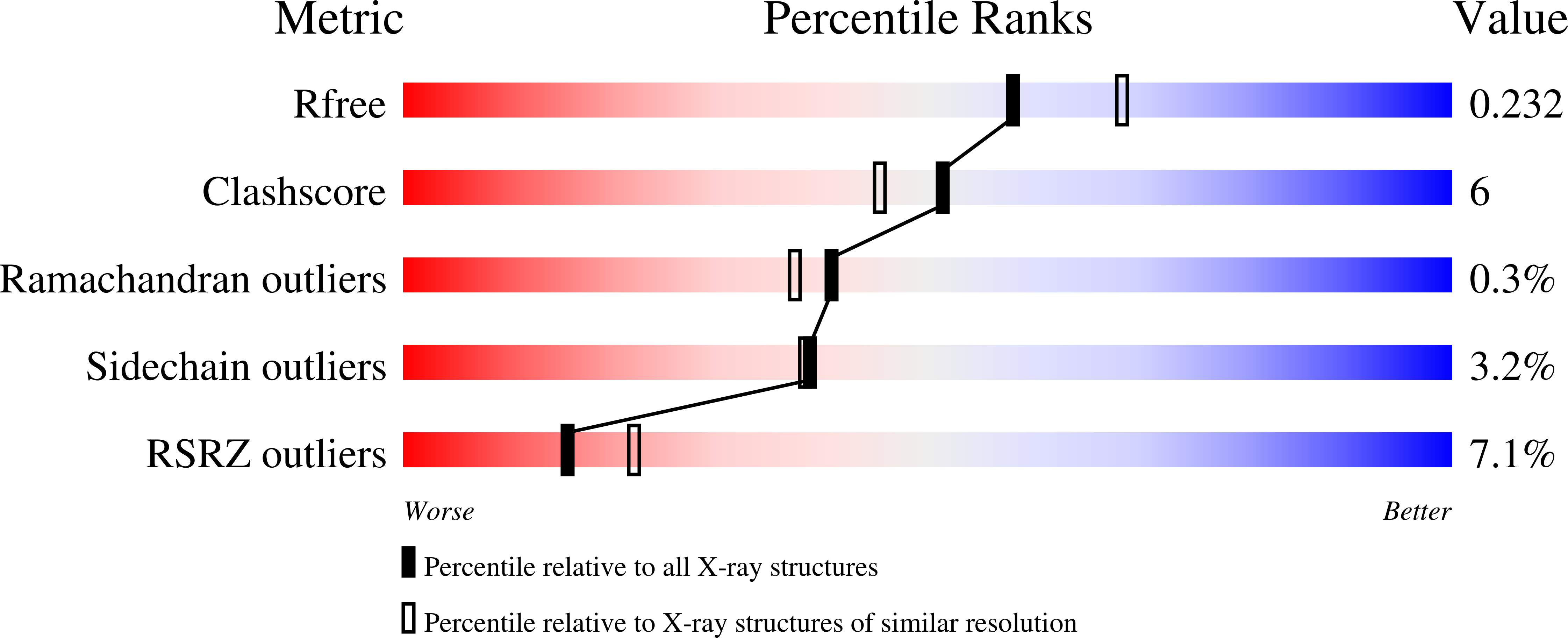
Quercetin 2,4-Dioxygenase Activates Dioxygen in a Side-On O2-Ni Complex.
Jeoung, J.H., Nianios, D., Fetzner, S., Dobbek, H.(2016) Angew Chem Int Ed Engl 55: 3281-3284
- PubMed: 26846734
- DOI: https://doi.org/10.1002/anie.201510741
- Primary Citation of Related Structures:
5FLH, 5FLI, 5FLJ - PubMed Abstract:
Quercetin 2,4-dioxygenase (quercetinase) from Streptomyces uses nickel as the active-site cofactor to catalyze oxidative cleavage of the flavonol quercetin. How this unusual active-site metal supports catalysis and O2 activation is under debate. We present crystal structures of Ni-quercetinase in three different states, thus providing direct insight into how quercetin and O2 are activated at the Ni(2+) ion. The Ni(2+) ion is coordinated by three histidine residues and a glutamate residue (E(76)) in all three states. Upon binding, quercetin replaces one water ligand at Ni and is stabilized by a short hydrogen bond through E(76) , the carboxylate group of which rotates by 90°. This conformational change weakens the interaction between Ni and the remaining water ligand, thereby preparing a coordination site at Ni to bind O2. O2 binds side-on to the Ni(2+) ion and is perpendicular to the C2-C3 and C3-C4 bonds of quercetin, which are cleaved in the following reaction steps.
Organizational Affiliation:
Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität zu Berlin, Unter den Linden 6, 10099, Berlin, Germany.