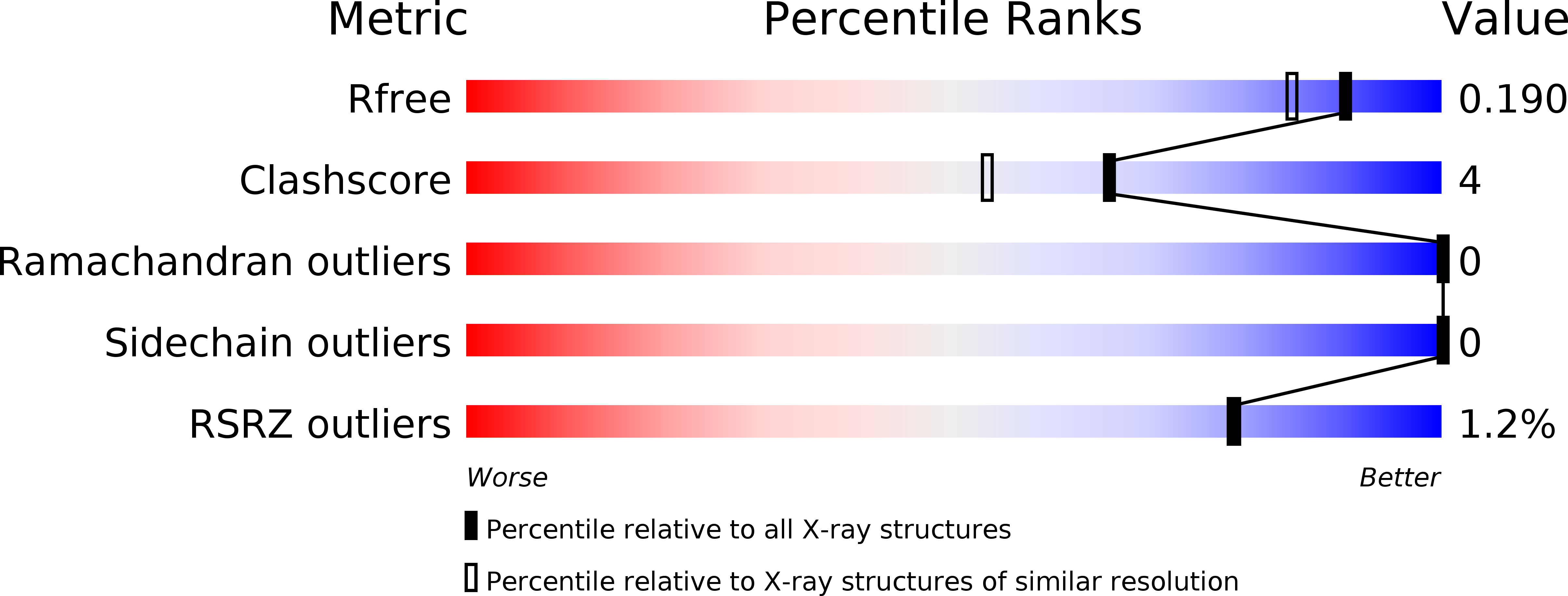
Determinants of oligosaccharide specificity of the carbohydrate-binding modules of AMP-activated protein kinase.
Mobbs, J.I., Koay, A., Di Paolo, A., Bieri, M., Petrie, E.J., Gorman, M.A., Doughty, L., Parker, M.W., Stapleton, D.I., Griffin, M.D., Gooley, P.R.(2015) Biochem J 468: 245-257
- PubMed: 25774984
- DOI: https://doi.org/10.1042/BJ20150270
- Primary Citation of Related Structures:
4Y0G, 4YEE, 4YEF - PubMed Abstract:
AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that is important in regulating energy metabolism in all eukaryotes. The β-subunit exists in two isoforms (β1 and β2) and contains a carbohydrate-binding module (CBM) that interacts with glycogen. The two CBM isoforms (β1- and β2-CBM) are near identical in sequence and structure, yet show differences in carbohydrate-binding affinity. β2-CBM binds linear carbohydrates with 4-fold greater affinity than β1-CBM and binds single α1,6-branched carbohydrates up to 30-fold tighter. To understand these affinity differences, especially for branched carbohydrates, we determined the NMR solution structure of β2-CBM in complex with the single α1,6-branched carbohydrate glucosyl-β-cyclodextrin (gBCD) which supported the dynamic nature of the binding site, but resonance broadening prevented defining where the α1,6 branch bound. We therefore solved the X-ray crystal structures of β1- and β2-CBM, in complex with gBCD, to 1.7 and 2.0 Å (1 Å=0.1 nm) respectively. The additional threonine (Thr101) of β2-CBM expands the size of the surrounding loop, creating a pocket that accommodates the α1,6 branch. Hydrogen bonds are formed between the α1,6 branch and the backbone of Trp99 and Lys102 side chain of β2-CBM. In contrast, the α1,6 branch could not be observed in the β1-CBM structure, suggesting that it does not form a specific interaction. The orientation of gBCD bound to β1- and β2-CBM is supported by thermodynamic and kinetic data obtained through isothermal titration calorimetry (ITC) and NMR. These results suggest that AMPK containing the muscle-specific β2-isoform may have greater affinity for partially degraded glycogen.
Organizational Affiliation:
*Department of Biochemistry and Molecular Biology, University of Melbourne, Parkville, Victoria 3010, Australia.