Structural basis for haem piracy from host haemopexin by Haemophilus influenzae.
Zambolin, S., Clantin, B., Chami, M., Hoos, S., Haouz, A., Villeret, V., Delepelaire, P.(2016) Nat Commun 7: 11590-11590
- PubMed: 27188378
- DOI: https://doi.org/10.1038/ncomms11590
- Primary Citation of Related Structures:
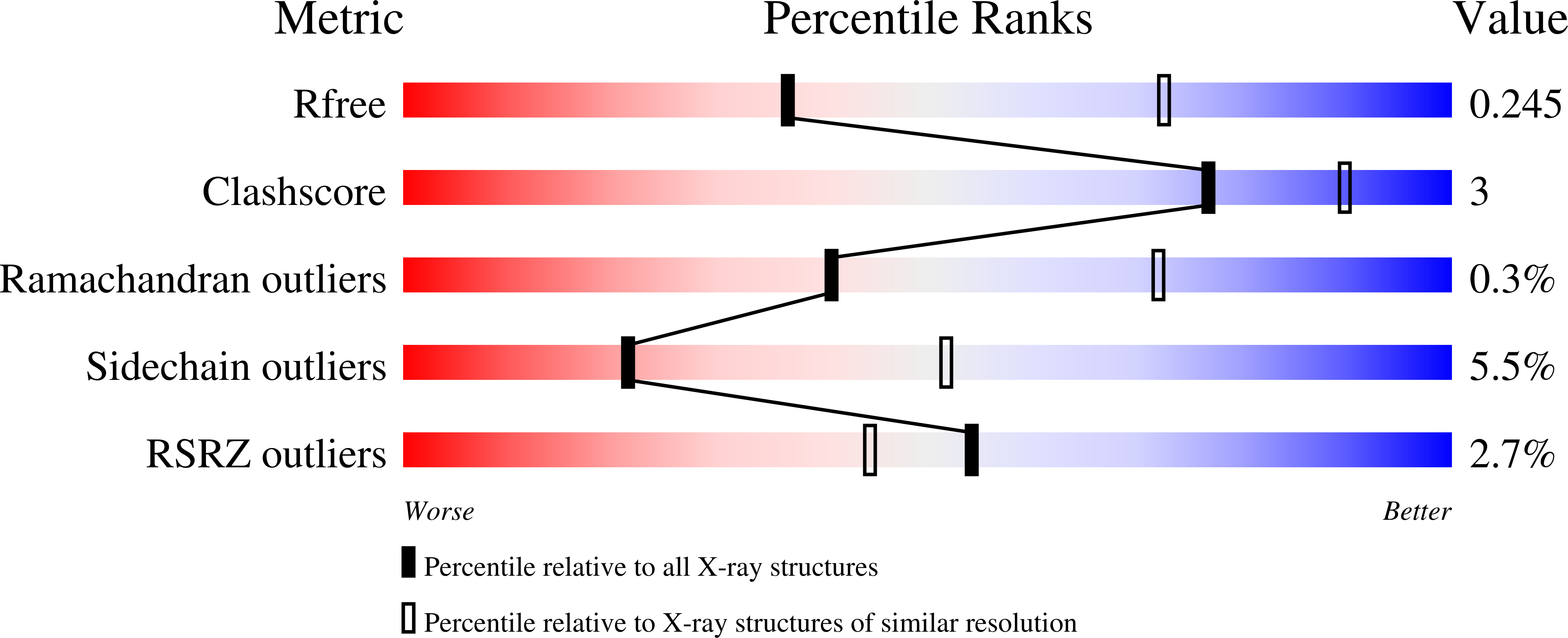
4RM6, 4RT6 - PubMed Abstract:
Haemophilus influenzae is an obligate human commensal/pathogen that requires haem for survival and can acquire it from several host haemoproteins, including haemopexin. The haem transport system from haem-haemopexin consists of HxuC, a haem receptor, and the two-partner-secretion system HxuB/HxuA. HxuA, which is exposed at the cell surface, is strictly required for haem acquisition from haemopexin. HxuA forms complexes with haem-haemopexin, leading to haem release and its capture by HxuC. The key question is how HxuA liberates haem from haemopexin. Here, we solve crystal structures of HxuA alone, and HxuA in complex with the N-terminal domain of haemopexin. A rational basis for the release of haem from haem-haemopexin is derived from both in vivo and in vitro studies. HxuA acts as a wedge that destabilizes the two-domains structure of haemopexin with a mobile loop on HxuA that favours haem ejection by redirecting key residues in the haem-binding pocket of haemopexin.
Organizational Affiliation:
CNRS, Université Paris 7 UMR 7099, Institut de Biologie Physico-Chimique, 13 rue Pierre et Marie Curie, 75005 Paris, France.