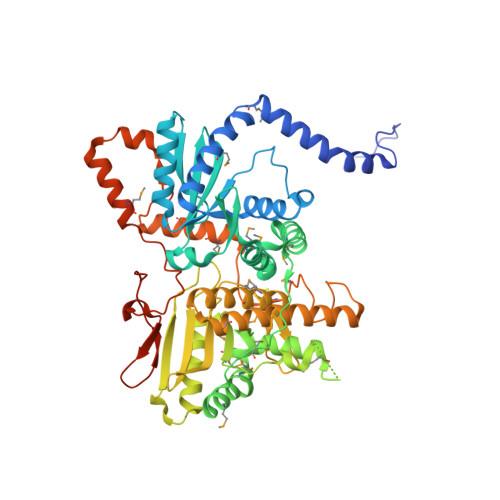
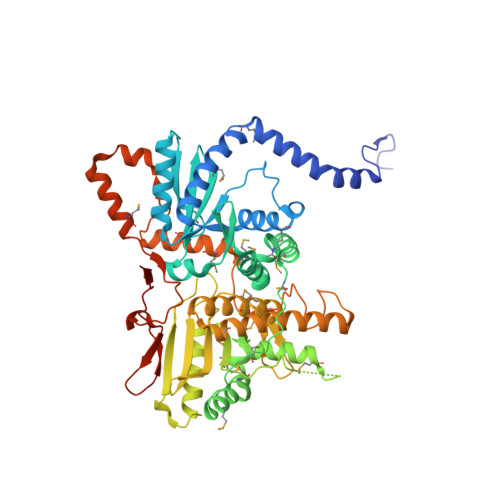
mRNA maturation in giant viruses: variation on a theme.
Priet, S., Lartigue, A., Debart, F., Claverie, J.M., Abergel, C.(2015) Nucleic Acids Res 43: 3776-3788
- PubMed: 25779049
- DOI: https://doi.org/10.1093/nar/gkv224
- Primary Citation of Related Structures:
4P37, 4WSE - PubMed Abstract:
Giant viruses from the Mimiviridae family replicate entirely in their host cytoplasm where their genes are transcribed by a viral transcription apparatus. mRNA polyadenylation uniquely occurs at hairpin-forming palindromic sequences terminating viral transcripts. Here we show that a conserved gene cluster both encode the enzyme responsible for the hairpin cleavage and the viral polyA polymerases (vPAP). Unexpectedly, the vPAPs are homodimeric and uniquely self-processive. The vPAP backbone structures exhibit a symmetrical architecture with two subdomains sharing a nucleotidyltransferase topology, suggesting that vPAPs originate from an ancestral duplication. A Poxvirus processivity factor homologue encoded by Megavirus chilensis displays a conserved 5'-GpppA 2'O methyltransferase activity but is also able to internally methylate the mRNAs' polyA tails. These findings elucidate how the arm wrestling between hosts and their viruses to access the translation machinery is taking place in Mimiviridae.
Organizational Affiliation:
Architecture et Fonction des Macromolécules Biologiques, CNRS UMR 7257, Aix-Marseille Université, 163 Avenue de Luminy, Case 932, 13288 Marseille cedex 9, France.