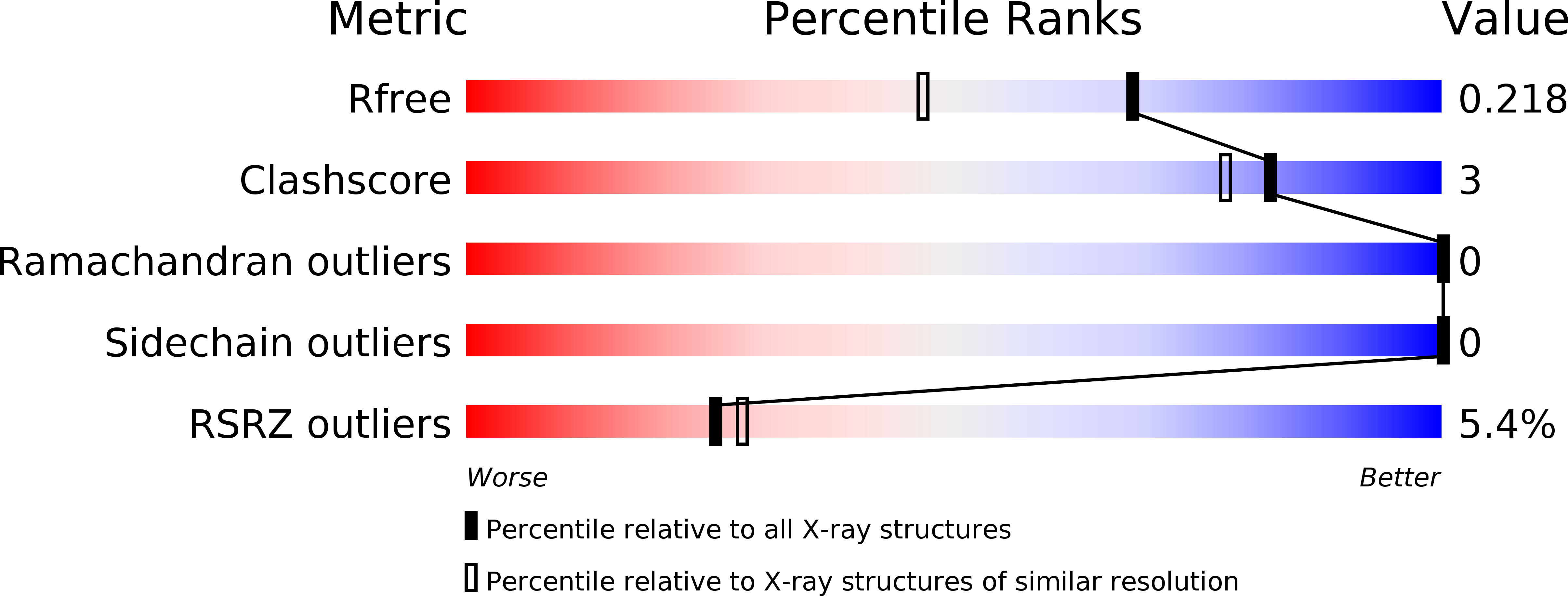
Structural basis for recognizing phosphoarginine and evolving residue-specific protein phosphatases in gram-positive bacteria.
Fuhrmann, J., Mierzwa, B., Trentini, D.B., Spiess, S., Lehner, A., Charpentier, E., Clausen, T.(2013) Cell Rep 3: 1832-1839
- PubMed: 23770242
- DOI: https://doi.org/10.1016/j.celrep.2013.05.023
- Primary Citation of Related Structures:
4KK3, 4KK4 - PubMed Abstract:
Many cellular pathways are regulated by the competing activity of protein kinases and phosphatases. The recent identification of arginine phosphorylation as a protein modification in bacteria prompted us to analyze the molecular basis of targeting phospho-arginine. In this work, we characterize an annotated tyrosine phosphatase, YwlE, that counteracts the protein arginine kinase McsB. Strikingly, structural studies of YwlE reaction intermediates provide a direct view on a captured arginine residue. Together with biochemical data, the crystal structures depict the evolution of a highly specific phospho-arginine phosphatase, with the use of a size-and-polarity filter for distinguishing phosphorylated arginine from other phosphorylated side chains. To confirm the proposed mechanism, we performed bioinformatic searches for phosphatases, employing a similar selectivity filter, and identified a protein in Drosophila melanogaster exhibiting robust arginine phosphatase activity. In sum, our findings uncover the molecular framework for specific targeting of phospho-arginine and suggest that protein arginine (de)phosphorylation may be relevant in eukaryotes.
Organizational Affiliation:
Research Institute of Molecular Pathology (IMP), A-1030 Vienna, Austria.