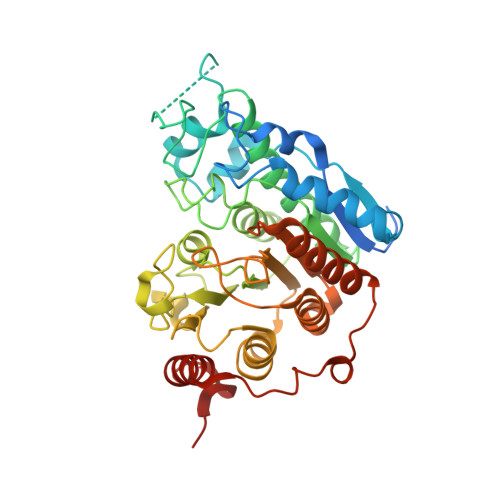
Selective Class Iia Histone Deacetylase Inhibition Via a Non-Chelating Zinc Binding Group
Lobera, M., Madauss, K.P., Pohlhaus, D.T., Wright, Q.G., Trocha, M., Schmidt, D.R., Baloglu, E., Trump, R.P., Head, M.S., Hofmann, G.A., Murray-Thompson, M., Schwartz, B., Chakravorty, S., Wu, Z., Mander, P.K., Kruidenier, L., Reid, R.A., Burkhart, W., Turunen, B.J., Rong, J.X., Wagner, C., Moyer, M.B., Wells, C., Hong, X., Moore, J.T., Williams, J.D., Soler, D., Ghosh, S., Nolan, M.A.(2013) Nat Chem Biol 9: 319
- PubMed: 23524983
- DOI: https://doi.org/10.1038/nchembio.1223
- Primary Citation of Related Structures:
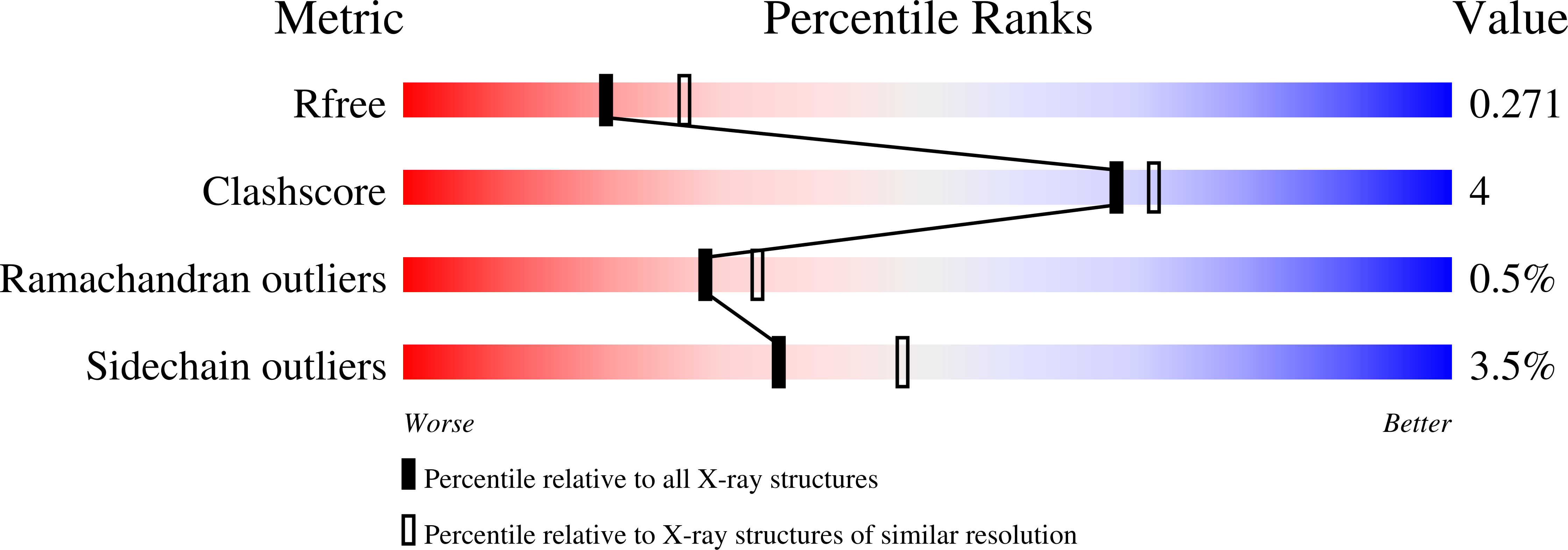
3ZNR, 3ZNS - PubMed Abstract:
In contrast to studies on class I histone deacetylase (HDAC) inhibitors, the elucidation of the molecular mechanisms and therapeutic potential of class IIa HDACs (HDAC4, HDAC5, HDAC7 and HDAC9) is impaired by the lack of potent and selective chemical probes. Here we report the discovery of inhibitors that fill this void with an unprecedented metal-binding group, trifluoromethyloxadiazole (TFMO), which circumvents the selectivity and pharmacologic liabilities of hydroxamates. We confirm direct metal binding of the TFMO through crystallographic approaches and use chemoproteomics to demonstrate the superior selectivity of the TFMO series relative to a hydroxamate-substituted analog. We further apply these tool compounds to reveal gene regulation dependent on the catalytic active site of class IIa HDACs. The discovery of these inhibitors challenges the design process for targeting metalloenzymes through a chelating metal-binding group and suggests therapeutic potential for class IIa HDAC enzyme blockers distinct in mechanism and application compared to current HDAC inhibitors.
Organizational Affiliation:
Tempero Pharmaceuticals, Cambridge, Massachusetts, USA.