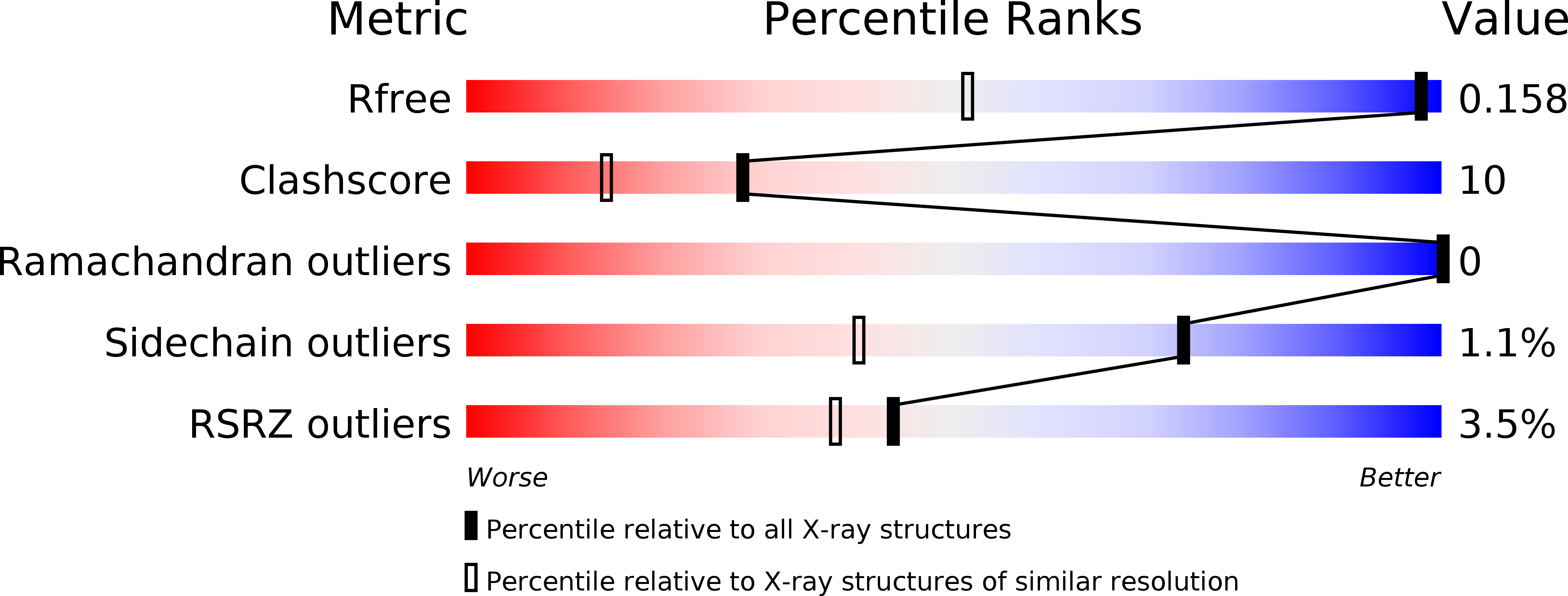
Structure of the RsbX phosphatase involved in the general stress response of Bacillus subtilis
Teh, A.H., Makino, M., Hoshino, T., Baba, S., Shimizu, N., Yamamoto, M., Kumasaka, T.(2015) Acta Crystallogr D Biol Crystallogr 71: 1392-1399
- PubMed: 26057679
- DOI: https://doi.org/10.1107/S1399004715007166
- Primary Citation of Related Structures:
3W40, 3W41, 3W42, 3W43, 3W44, 3W45 - PubMed Abstract:
In the general stress response of Bacillus subtilis, which is governed by the sigma factor σ(B), stress signalling is relayed by a cascade of Rsb proteins that regulate σ(B) activity. RsbX, a PPM II phosphatase, halts the response by dephosphorylating the stressosome composed of RsbR and RsbS. The crystal structure of RsbX reveals a reorganization of the catalytic centre, with the second Mn(2+) ion uniquely coordinated by Gly47 O from the β4-α1 loop instead of a water molecule as in PPM I phosphatases. An extra helical turn of α1 tilts the loop towards the metal-binding site, and the β2-β3 loop swings outwards to accommodate this tilting. The residues critical for this defining feature of the PPM II phosphatases are highly conserved. Formation of the catalytic centre is metal-specific, as crystallization with Mg(2+) ions resulted in a shift of the β4-α1 loop that led to loss of the second ion. RsbX also lacks the flap subdomain characteristic of PPM I phosphatases. On the basis of a stressosome model, the activity of RsbX towards RsbR-P and RsbS-P may be influenced by the different accessibilities of their phosphorylation sites.
Organizational Affiliation:
Japan Synchrotron Radiation Research Institute (JASRI/SPring-8), 1-1-1 Kouto, Sayo, Hyogo 679-5198, Japan.