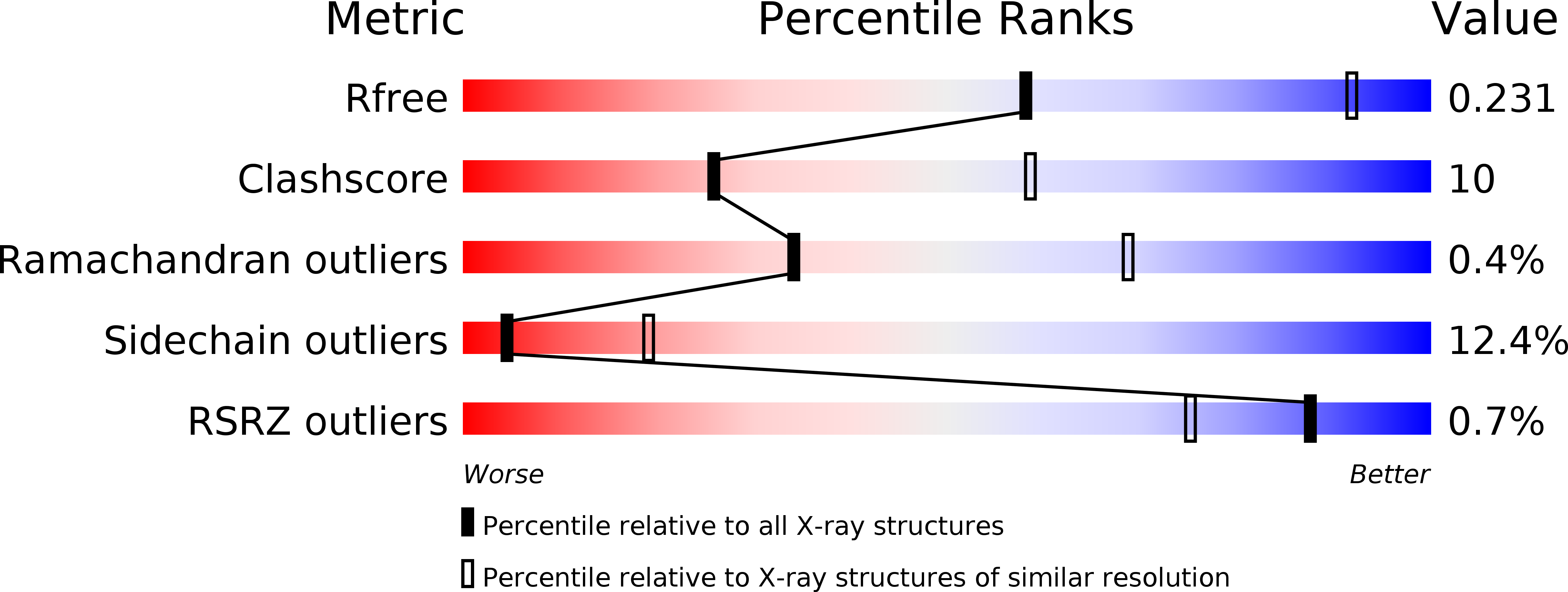
Structural Basis for the Transcriptional Regulation of Heme Homeostasis in Lactococcus lactis.
Sawai, H., Yamanaka, M., Sugimoto, H., Shiro, Y., Aono, S.(2012) J Biol Chem 287: 30755-30768
- PubMed: 22798069
- DOI: https://doi.org/10.1074/jbc.M112.370916
- Primary Citation of Related Structures:
3VOK, 3VOX, 3VP5 - PubMed Abstract:
Although heme is a crucial element for many biological processes including respiration, heme homeostasis should be regulated strictly due to the cytotoxicity of free heme molecules. Numerous lactic acid bacteria, including Lactococcus lactis, acquire heme molecules exogenously to establish an aerobic respiratory chain. A heme efflux system plays an important role for heme homeostasis to avoid cytotoxicity of acquired free heme, but its regulatory mechanism is not clear. Here, we report that the transcriptional regulator heme-regulated transporter regulator (HrtR) senses and binds a heme molecule as its physiological effector to regulate the expression of the heme-efflux system responsible for heme homeostasis in L. lactis. To elucidate the molecular mechanisms of how HrtR senses a heme molecule and regulates gene expression for the heme efflux system, we determined the crystal structures of the apo-HrtR·DNA complex, apo-HrtR, and holo-HrtR at a resolution of 2.0, 3.1, and 1.9 Å, respectively. These structures revealed that HrtR is a member of the TetR family of transcriptional regulators. The residue pair Arg-46 and Tyr-50 plays a crucial role for specific DNA binding through hydrogen bonding and a CH-π interaction with the DNA bases. HrtR adopts a unique mechanism for its functional regulation upon heme sensing. Heme binding to HrtR causes a coil-to-helix transition of the α4 helix in the heme-sensing domain, which triggers a structural change of HrtR, causing it to dissociate from the target DNA for derepression of the genes encoding the heme efflux system. HrtR uses a unique heme-sensing motif with bis-His (His-72 and His-149) ligation to the heme, which is essential for the coil-to-helix transition of the α4 helix upon heme sensing.
Organizational Affiliation:
Okazaki Institute for Integrative Bioscience and Institute for Molecular Science, National Institutes of Natural Sciences, 5-1 Higashiyama, Myodaiji, Okazaki 444-8787, Japan.