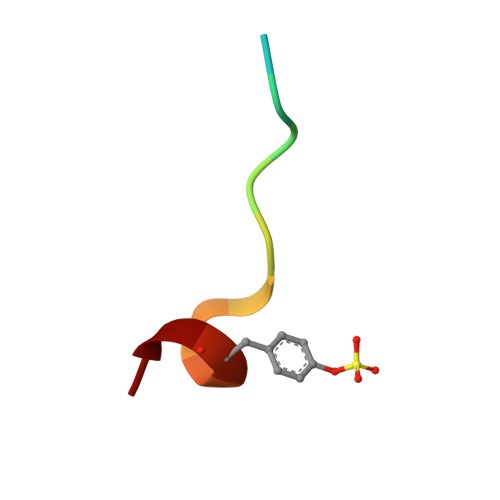
Ligand binding stepwise disrupts water network in thrombin: enthalpic and entropic changes reveal classical hydrophobic effect
Biela, A., Sielaff, F., Terwesten, F., Heine, A., Steinmetzer, T., Klebe, G.(2012) J Med Chem 55: 6094-6110
- PubMed: 22612268
- DOI: https://doi.org/10.1021/jm300337q
- Primary Citation of Related Structures:
3RLW, 3RLY, 3RM0, 3RM2, 3RML, 3RMM, 3RMN, 3RMO, 3T5F, 3UWJ - PubMed Abstract:
Well-ordered water molecules are displaced from thrombin's hydrophobic S3/4-pocket by P3-varied ligands (Gly, d-Ala, d-Val, d-Leu to d-Cha with increased hydrophobicity and steric requirement). Two series with 2-(aminomethyl)-5-chlorobenzylamide and 4-amidinobenzylamide at P1 were examined by ITC and crystallography. Although experiencing different interactions in S1, they display almost equal potency. For both scaffolds the terminal benzylsulfonyl substituent differs in binding, whereas the increasingly bulky P3-groups address S3/4 pocket similarly. Small substituents leave the solvation pattern unperturbed as found in the uncomplexed enzyme while increasingly larger ones stepwise displace the waters. Medium-sized groups show patterns with partially occupied waters. The overall 40-fold affinity enhancement correlates with water displacement and growing number of van der Waals contacts and is mainly attributed to favorable entropy. Both Gly derivatives deviate from the series and adopt different binding modes. Nonetheless, their thermodynamic signatures are virtually identical with the homologous d-Ala derivatives. Accordingly, unchanged thermodynamic profiles are no reliable indicator for conserved binding modes.
Organizational Affiliation:
Department of Pharmaceutical Chemistry, Philipps University Marburg, Marbacher Weg 6, 35032 Marburg, Germany.