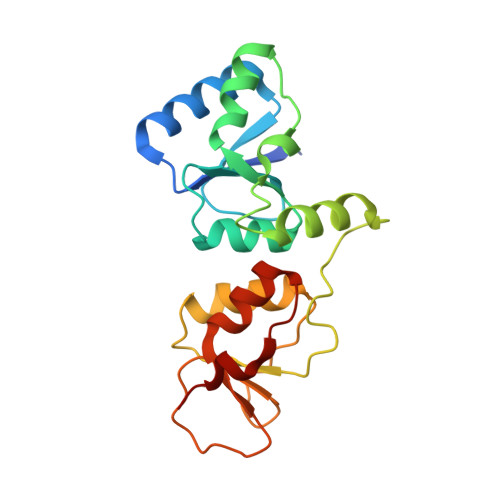
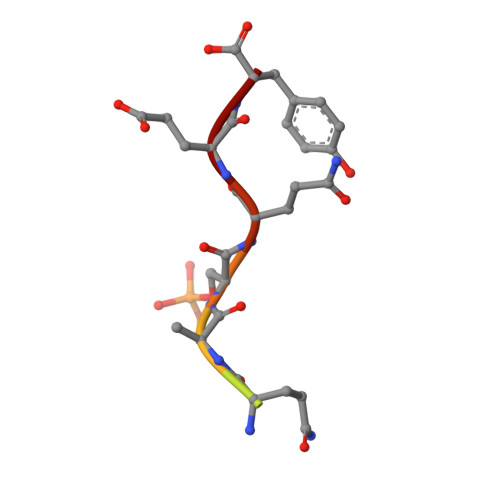
Dual recognition of phosphoserine and phosphotyrosine in histone variant H2A.X by DNA damage response protein MCPH1.
Singh, N., Basnet, H., Wiltshire, T.D., Mohammad, D.H., Thompson, J.R., Heroux, A., Botuyan, M.V., Yaffe, M.B., Couch, F.J., Rosenfeld, M.G., Mer, G.(2012) Proc Natl Acad Sci U S A 109: 14381-14386
- PubMed: 22908299
- DOI: https://doi.org/10.1073/pnas.1212366109
- Primary Citation of Related Structures:
3SZM, 3U3Z - PubMed Abstract:
Tyr142, the C-terminal amino acid of histone variant H2A.X is phosphorylated by WSTF (Williams-Beuren syndrome transcription factor), a component of the WICH complex (WSTF-ISWI chromatin-remodeling complex), under basal conditions in the cell. In response to DNA double-strand breaks (DSBs), H2A.X is instantaneously phosphorylated at Ser139 by the kinases ATM and ATR and is progressively dephosphorylated at Tyr142 by the Eya1 and Eya3 tyrosine phosphatases, resulting in a temporal switch from a postulated diphosphorylated (pSer139, pTyr142) to monophosphorylated (pSer139) H2A.X state. How mediator proteins interpret these two signals remains a question of fundamental interest. We provide structural, biochemical, and cellular evidence that Microcephalin (MCPH1), an early DNA damage response protein, can read both modifications via its tandem BRCA1 C-terminal (BRCT) domains, thereby emerging as a versatile sensor of H2A.X phosphorylation marks. We show that MCPH1 recruitment to sites of DNA damage is linked to both states of H2A.X.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN 55905, USA.