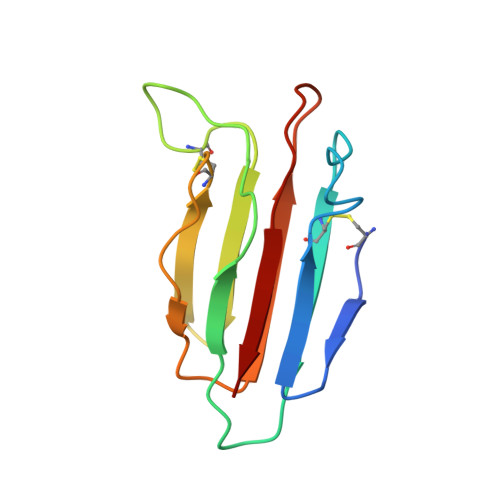
Structural Basis of Mannan-Binding Lectin Recognition by Its Associated Serine Protease MASP-1: Implications for Complement Activation.
Gingras, A.R., Girija, U.V., Keeble, A.H., Panchal, R., Mitchell, D.A., Moody, P.C., Wallis, R.(2011) Structure 19: 1635-1643
- PubMed: 22078562
- DOI: https://doi.org/10.1016/j.str.2011.08.014
- Primary Citation of Related Structures:
3POB, 3POD, 3POE, 3POF, 3POG, 3POI, 3POJ, 3PON - PubMed Abstract:
Complement activation contributes directly to health and disease. It neutralizes pathogens and stimulates immune processes. Defects lead to immunodeficiency and autoimmune diseases, whereas inappropriate activation causes self-damage. In the lectin and classical pathways, complement is triggered upon recognition of a pathogen by an activating complex. Here we present the first structure of such a complex in the form of the collagen-like domain of mannan-binding lectin (MBL) and the binding domain of its associated protease (MASP-1/-3). The collagen binds within a groove using a pivotal lysine side chain that interacts with Ca(2+)-coordinating residues, revealing the essential role of Ca(2+). This mode of binding is prototypic for all activating complexes of the lectin and classical pathways, and suggests a general mechanism for the global changes that drive activation. The structural insights reveal a new focus for inhibitors and we have validated this concept by targeting the binding pocket of the MASP.
Organizational Affiliation:
Department of Biochemistry, University of Leicester, Leicester, LE1 9HN, UK.