ClbP x-ray structure, the prototype of a peptidase family associated to NRP production
Dubois, D., Baron, O., Cougnoux, A., Delmas, J., Pradel, N., Nougayrede, J.P., Robin, F., Oswald, E., Bonnet, R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| beta-lactamase | 335 | Escherichia coli IHE3034 | Mutation(s): 0 Gene Names: clbp, ECOK1_2171 EC: 3.5.2.6 | 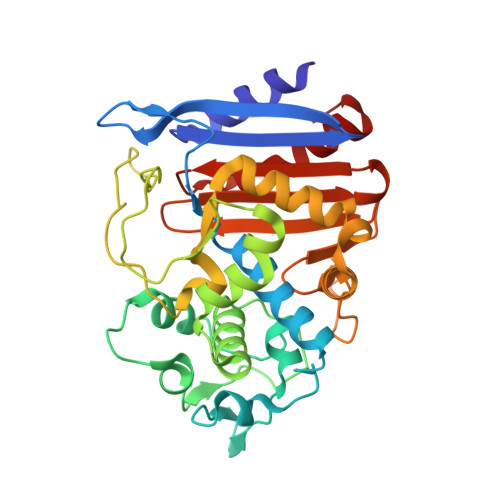 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 103.94 | α = 90 |
| b = 149.93 | β = 123.9 |
| c = 87.33 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DNA | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |