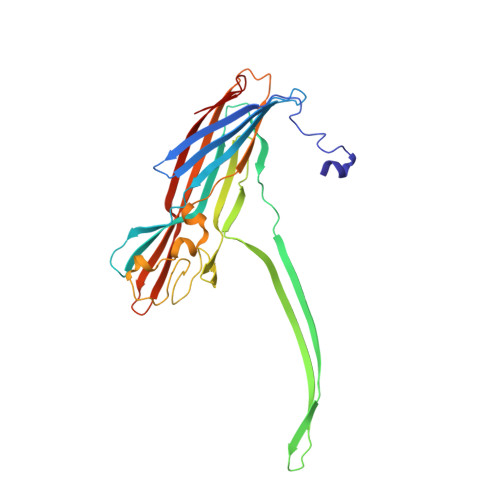
Molecular bases of cyclodextrin adapter interactions with engineered protein nanopores.
Banerjee, A., Mikhailova, E., Cheley, S., Gu, L.Q., Montoya, M., Nagaoka, Y., Gouaux, E., Bayley, H.(2010) Proc Natl Acad Sci U S A 107: 8165-8170
- PubMed: 20400691
- DOI: https://doi.org/10.1073/pnas.0914229107
- Primary Citation of Related Structures:
3M2L, 3M3R, 3M4D, 3M4E - PubMed Abstract:
Engineered protein pores have several potential applications in biotechnology: as sensor elements in stochastic detection and ultrarapid DNA sequencing, as nanoreactors to observe single-molecule chemistry, and in the construction of nano- and micro-devices. One important class of pores contains molecular adapters, which provide internal binding sites for small molecules. Mutants of the alpha-hemolysin (alphaHL) pore that bind the adapter beta-cyclodextrin (betaCD) approximately 10(4) times more tightly than the wild type have been obtained. We now use single-channel electrical recording, protein engineering including unnatural amino acid mutagenesis, and high-resolution x-ray crystallography to provide definitive structural information on these engineered protein nanopores in unparalleled detail.
Organizational Affiliation:
Department of Chemistry, University of Oxford, Oxford, OX1 3TA, United Kingdom.