Structural insight into methyl-coenzyme M reductase chemistry using coenzyme B analogues.
Cedervall, P.E., Dey, M., Pearson, A.R., Ragsdale, S.W., Wilmot, C.M.(2010) Biochemistry 49: 7683-7693
- PubMed: 20707311
- DOI: https://doi.org/10.1021/bi100458d
- Primary Citation of Related Structures:
3M1V, 3M2R, 3M2U, 3M2V, 3M30, 3M32 - PubMed Abstract:
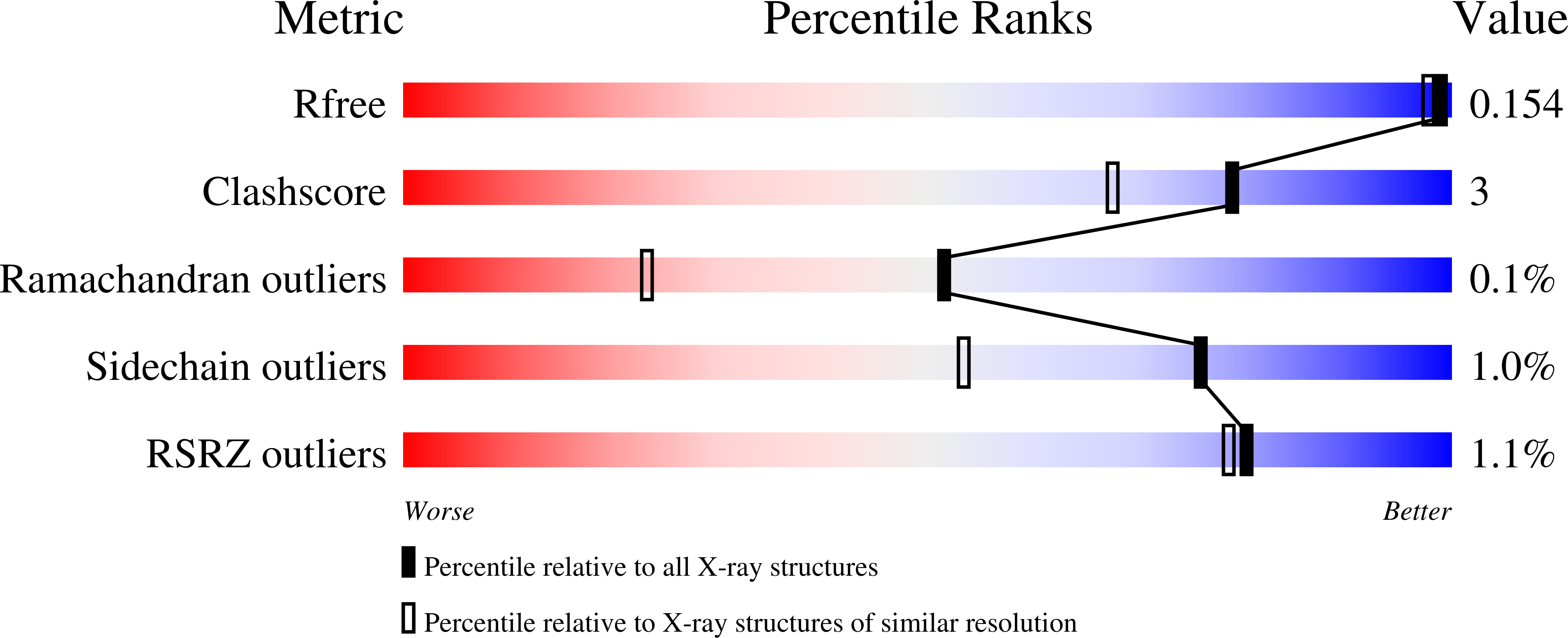
Methyl-coenzyme M reductase (MCR) catalyzes the final and rate-limiting step in methane biogenesis: the reduction of methyl-coenzyme M (methyl-SCoM) by coenzyme B (CoBSH) to methane and a heterodisulfide (CoBS-SCoM). Crystallographic studies show that the active site is deeply buried within the enzyme and contains a highly reduced nickel-tetrapyrrole, coenzyme F(430). Methyl-SCoM must enter the active site prior to CoBSH, as species derived from methyl-SCoM are always observed bound to the F(430) nickel in the deepest part of the 30 A long substrate channel that leads from the protein surface to the active site. The seven-carbon mercaptoalkanoyl chain of CoBSH binds within a 16 A predominantly hydrophobic part of the channel close to F(430), with the CoBSH thiolate lying closest to the nickel at a distance of 8.8 A. It has previously been suggested that binding of CoBSH initiates catalysis by inducing a conformational change that moves methyl-SCoM closer to the nickel promoting cleavage of the C-S bond of methyl-SCoM. In order to better understand the structural role of CoBSH early in the MCR mechanism, we have determined crystal structures of MCR in complex with four different CoBSH analogues: pentanoyl, hexanoyl, octanoyl, and nonanoyl derivatives of CoBSH (CoB(5)SH, CoB(6)SH, CoB(8)SH, and CoB(9)SH, respectively). The data presented here reveal that the shorter CoB(5)SH mercaptoalkanoyl chain overlays with that of CoBSH but terminates two units short of the CoBSH thiolate position. In contrast, the mercaptoalkanoyl chain of CoB(6)SH adopts a different conformation, such that its thiolate is coincident with the position of the CoBSH thiolate. This is consistent with the observation that CoB(6)SH is a slow substrate. A labile water in the substrate channel was found to be a sensitive indicator for the presence of CoBSH and HSCoM. The longer CoB(8)SH and CoB(9)SH analogues can be accommodated in the active site through exclusion of this water. These analogues react with Ni(III)-methyl, a proposed MCR catalytic intermediate of methanogenesis. The CoB(8)SH thiolate is 2.6 A closer to the nickel than that of CoBSH, but the additional carbon of CoB(9)SH only decreases the nickel thiolate distance a further 0.3 A. Although the analogues do not induce any structural changes in the substrate channel, the thiolates appear to preferentially bind at two distinct positions in the channel, one being the previously observed CoBSH thiolate position and the other being at a hydrophobic annulus of residues that lines the channel proximal to the nickel.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, Minnesota 55455, USA.