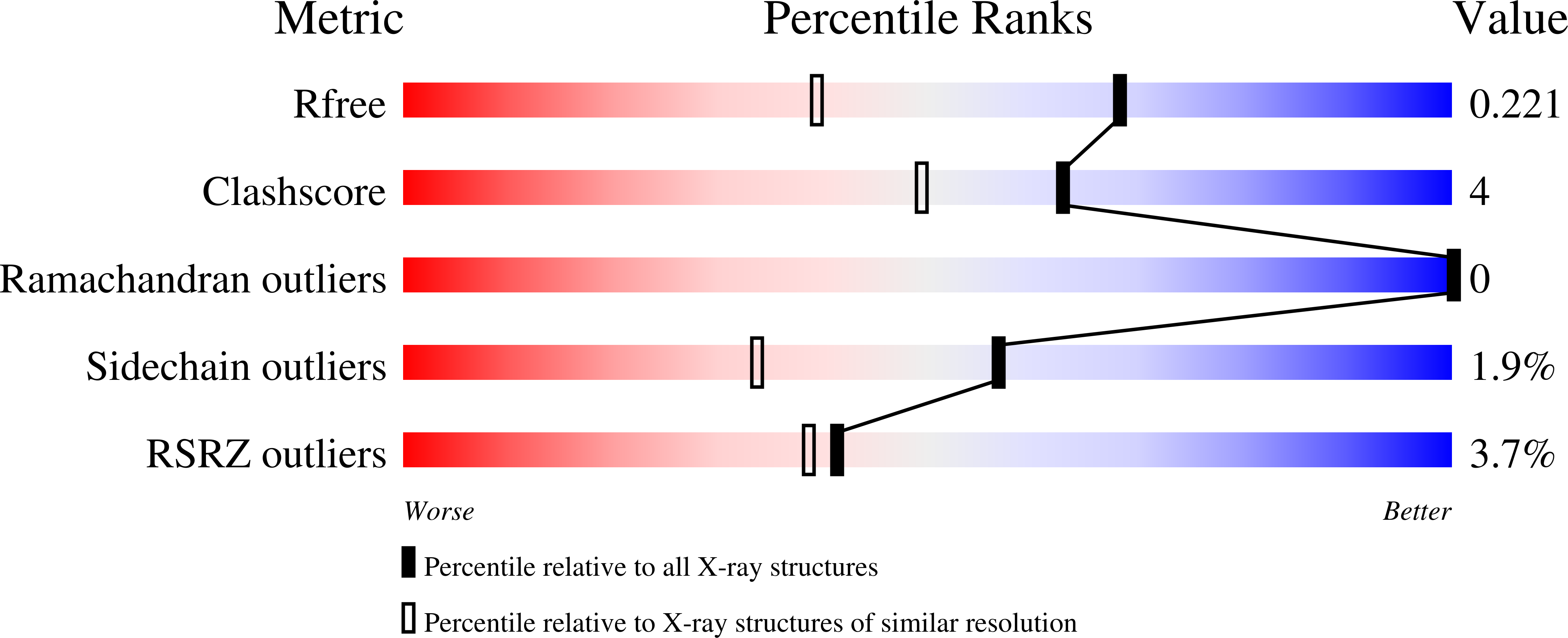
Structural characterization of mutations at the oxygen activation site in monomeric sarcosine oxidase.
Jorns, M.S., Chen, Z.W., Mathews, F.S.(2010) Biochemistry 49: 3631-3639
- PubMed: 20353187
- DOI: https://doi.org/10.1021/bi100160j
- Primary Citation of Related Structures:
3M0O, 3M12, 3M13 - PubMed Abstract:
Oxygen reduction and sarcosine oxidation in monomeric sarcosine oxidase (MSOX) occur at separate sites above the si- and re-faces, respectively, of the flavin ring. Mutagenesis studies implicate Lys265 as the oxygen activation site. Substitution of Lys265 with a neutral (Met, Gln, or Ala) or basic (Arg) residue results in an approximately 10(4)- or 250-fold decrease, respectively, in the reaction rate. The overall structure of MSOX and residue conformation in the sarcosine binding cavity are unaffected by replacement of Lys265 with Met or Arg. The side chain of Met265 exhibits the same configuration in each molecule of Lys265Met crystals and is nearly congruent with Lys265 in wild-type MSOX. The side chain of Arg265 is, however, dramatically shifted ( approximately 4-5 A) compared with Lys265, points in the opposite direction, and exhibits significant conformational variability between molecules of the same crystal. The major species in solutions of Lys265Arg is likely to contain a "flipped-out" Arg265 and exhibit negligible oxygen activation, similar to Lys265Met. The 400-fold higher oxygen reactivity observed with Lys265Arg is attributed to a minor (<1%) "flipped-in" Arg265 conformer whose oxygen reactivity is similar to that of wild-type MSOX. A structural water (WAT1), found above the si-face of the flavin ring in all previously determined MSOX structures, is part of an apparent proton relay system that extends from FAD N(5) to bulk solvent. WAT1 is strikingly absent in Lys265Met and Lys265Arg, a feature that may account for the apparent kinetic stabilization of a reductive half-reaction intermediate that is detectable with the mutants but not wild-type MSOX.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, Pennsylvania 19102, USA. marilyn.jorns@drexelmed.edu