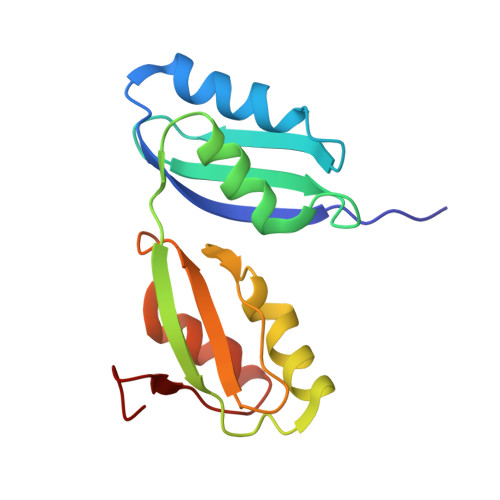
Structure and Cu(I)-binding properties of the N-terminal soluble domains of Bacillus subtilis CopA
Singleton, C., Banci, L., Ciofi-Baffoni, S., Tenori, L., Kihlken, M.A., Boetzel, R., Le Brun, N.E.(2008) Biochem J 411: 571-579
- PubMed: 18215122
- DOI: https://doi.org/10.1042/BJ20071620
- Primary Citation of Related Structures:
2RML - PubMed Abstract:
CopA, a P-type ATPase from Bacillus subtilis, plays a major role in the resistance of the cell to copper by effecting the export of the metal across the cytoplasmic membrane. The N-terminus of the protein features two soluble domains (a and b), that each contain a Cu(I)-binding motif, MTCAAC. We have generated a stable form of the wild-type two-domain protein, CopAab, and determined its solution structure. This was found to be similar to that reported previously for a higher stability S46V variant, with minor differences mostly confined to the Ser(46)-containing beta3-strand of domain a. Chemical-shift analysis demonstrated that the two Cu(I)-binding motifs, located at different ends of the protein molecule, are both able to participate in Cu(I) binding and that Cu(I) is in rapid exchange between protein molecules. Surprisingly, UV-visible and fluorescence spectroscopy indicate very different modes of Cu(I) binding below and above a level of 1 Cu(I) per protein, consistent with a major structural change occurring above 1 Cu(I) per CopAab. Analytical equilibrium centrifugation and gel filtration results show that this is a result of Cu(I)-mediated dimerization of the protein. The resulting species is highly luminescent, indicating the presence of a solvent-shielded Cu(I) cluster.
Organizational Affiliation:
Centre for Metalloprotein Spectroscopy and Biology, School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich NR4 7TJ, UK.