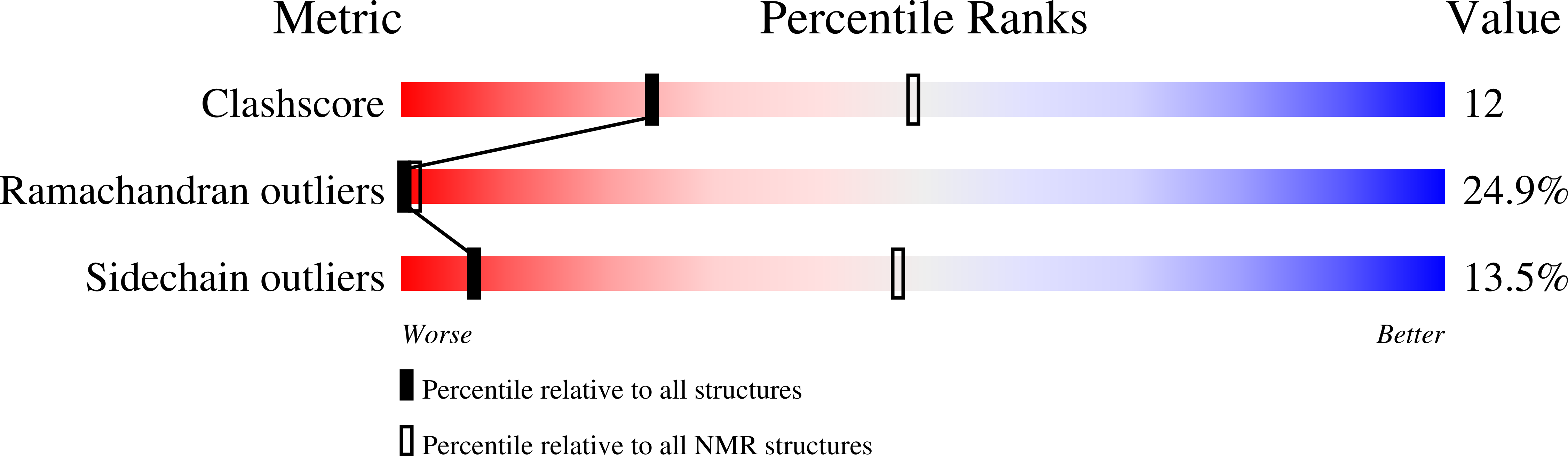PSCD Domains of Pleuralin-1 from the Diatom Cylindrotheca fusiformis: NMR Structures and Interactions with Other Biosilica-Associated Proteins.
De Sanctis, S., Wenzler, M., Kroger, N., Malloni, W.M., Sumper, M., Deutzmann, R., Zadravec, P., Brunner, E., Kremer, W., Kalbitzer, H.R.(2016) Structure 24: 1178-1191
- PubMed: 27320836
- DOI: https://doi.org/10.1016/j.str.2016.04.021
- Primary Citation of Related Structures:
2MK0, 2NBI - PubMed Abstract:
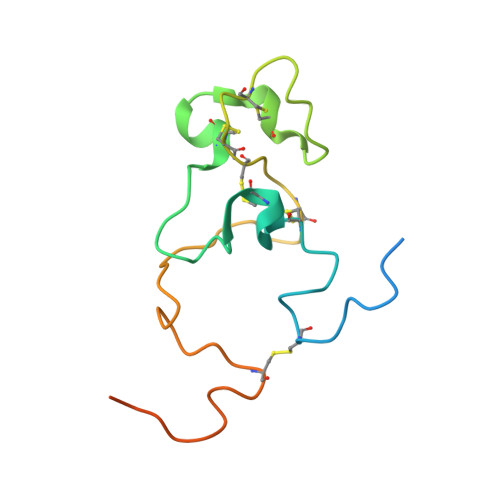
Diatoms are eukaryotic unicellular algae characterized by silica cell walls and associated with three unique protein families, the pleuralins, frustulins, and silaffins. The NMR structure of the PSCD4 domain of pleuralin-1 from Cylindrotheca fusiformis contains only three short helical elements and is stabilized by five unique disulfide bridges. PSCD4 contains two binding sites for Ca(2+) ions with millimolar affinity. NMR-based interaction studies show an interaction of the domain with native silaffin-1A as well as with α-frustulins. The interaction sites of the two proteins mapped on the PSCD4 structure are contiguous and show only a small overlap. A plausible functional role of pleuralin could be to bind simultaneously silaffin-1A located inside the cell wall and α-frustulin coating the cell wall, thus connecting the interfaces between hypotheca and epitheca at the girdle bands. Restrained molecular dynamics calculations suggest a bead-chain-like structure of the central part of pleuralin-1.
Organizational Affiliation:
Institute of Biophysics und Physical Biochemistry, Centre of Magnetic Resonance in Chemistry and Biomedicine, University of Regensburg, 93040 Regensburg, Germany.