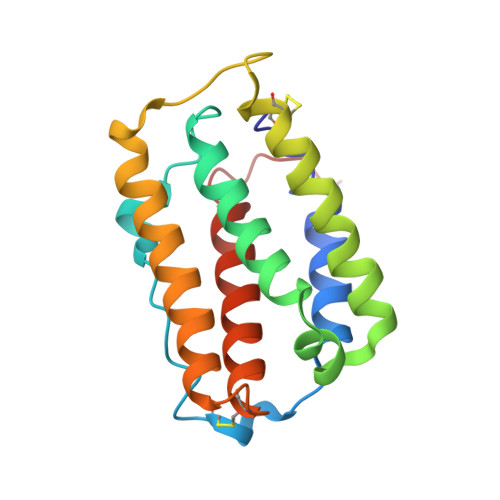
A single N-acetylgalactosamine residue at threonine 106 modifies the dynamics and structure of interferon alpha2a around the glycosylation site.
Ghasriani, H., Belcourt, P.J., Sauve, S., Hodgson, D.J., Brochu, D., Gilbert, M., Aubin, Y.(2013) J Biol Chem 288: 247-254
- PubMed: 23184955
- DOI: https://doi.org/10.1074/jbc.M112.413252
- Primary Citation of Related Structures:
2LMS - PubMed Abstract:
Enzymatic addition of GalNAc to isotopically labeled IFNα2a produced in Escherichia coli yielded the O-linked glycoprotein GalNAcα-[(13)C,(15)N]IFNα2a. The three-dimensional structure of GalNAcα-IFNα2a has been determined in solution by NMR spectroscopy at high resolution. Proton-nitrogen heteronuclear Overhauser enhancement measurements revealed that the addition of a single monosaccharide unit at Thr-106 significantly slowed motions of the glycosylation loop on the nanosecond time scale. Subsequent addition of a Gal unit produced Gal(β1,3)GalNAcα-[(13)C,(15)N]IFNα2a. This extension resulted in a further decrease in the dynamics of this loop. The methodology used here allowed the first such description of the structure and dynamics of an O-glycoprotein and opens the way to the study of this class of proteins.
Organizational Affiliation:
Centre for Vaccine Evaluation, Biologics and Genetic Therapies Directorate, Health Canada, Ottawa, Ontario K1A 0K9, Canada.