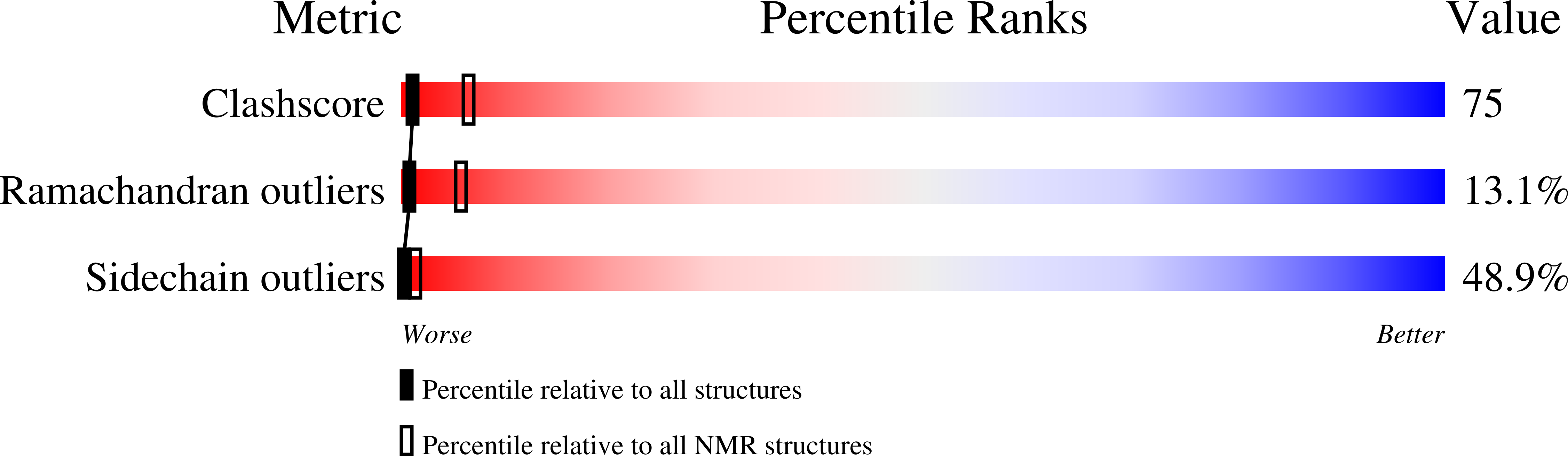Solution structure of the Equine Infectious Anemia Virus p9 protein: a rationalization of its different ALIX binding requirements compared to the analogous HIV-p6 protein
Sharma, A., Bruns, K., Roder, R., Henklein, P., Votteler, J., Wray, V., Schubert, U.(2009) BMC Struct Biol 9: 74-74
- PubMed: 20015412
- DOI: https://doi.org/10.1186/1472-6807-9-74
- Primary Citation of Related Structures:
2K84 - PubMed Abstract:
The equine infection anemia virus (EIAV) p9 Gag protein contains the late (L-) domain required for efficient virus release of nascent virions from the cell membrane of infected cell. In the present study the p9 protein and N- and C-terminal fragments (residues 1-21 and 22-51, respectively) were chemically synthesized and used for structural analyses. Circular dichroism and 1H-NMR spectroscopy provide the first molecular insight into the secondary structure and folding of this 51-amino acid protein under different solution conditions. Qualitative 1H-chemical shift and NOE data indicate that in a pure aqueous environment p9 favors an unstructured state. In its most structured state under hydrophobic conditions, p9 adopts a stable helical structure within the C-terminus. Quantitative NOE data further revealed that this alpha-helix extends from Ser-27 to Ser-48, while the N-terminal residues remain unstructured. The structural elements identified for p9 differ substantially from that of the functional homologous HIV-1 p6 protein. These structural differences are discussed in the context of the different types of L-domains regulating distinct cellular pathways in virus budding. EIAV p9 mediates virus release by recruiting the ALG2-interacting protein X (ALIX) via the YPDL-motif to the site of virus budding, the counterpart of the YPXnL-motif found in p6. However, p6 contains an additional PTAP L-domain that promotes HIV-1 release by binding to the tumor susceptibility gene 101 (Tsg101). The notion that structures found in p9 differ form that of p6 further support the idea that different mechanisms regulate binding of ALIX to primary versus secondary L-domains types.
Organizational Affiliation:
Department of Structural Biology, Helmholtz Centre for Infection Research, D-38124 Braunschweig, Germany. alok.sharma@gmail.com