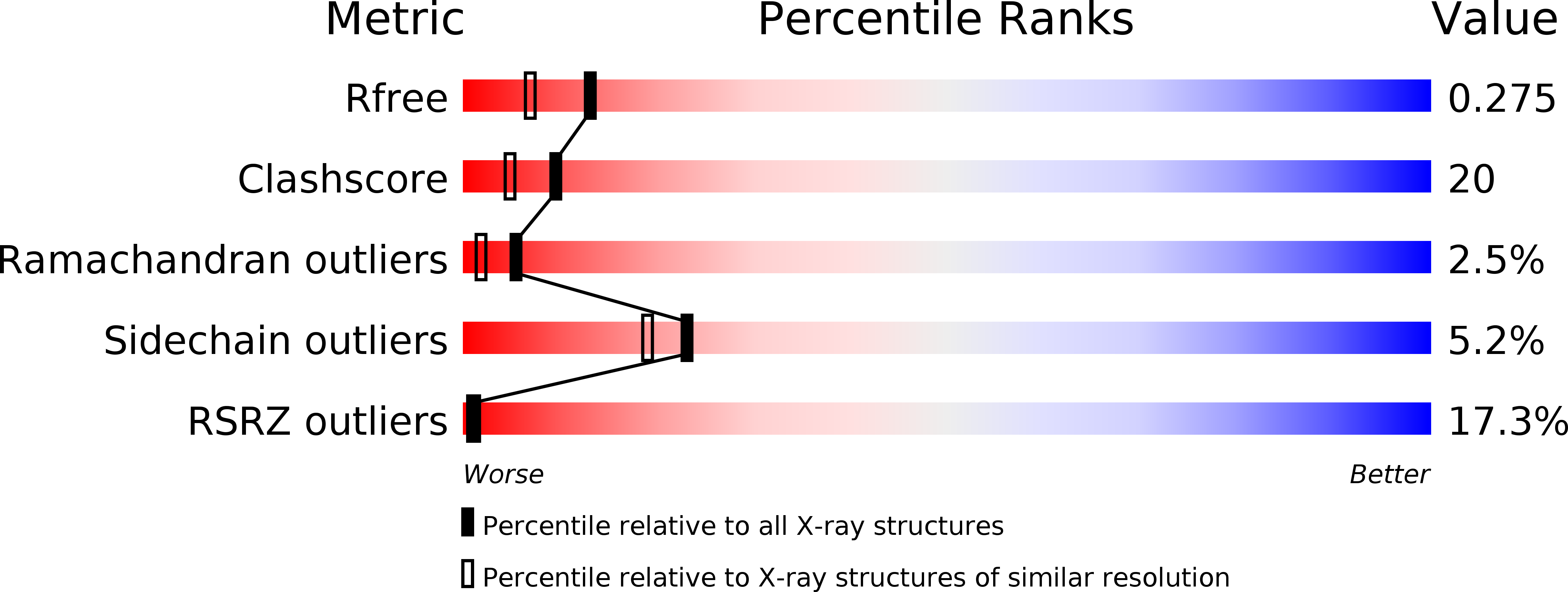
Structural mechanism and photoprotective function of water-soluble chlorophyll-binding protein.
Horigome, D., Satoh, H., Itoh, N., Mitsunaga, K., Oonishi, I., Nakagawa, A., Uchida, A.(2007) J Biol Chem 282: 6525-6531
- PubMed: 17170107
- DOI: https://doi.org/10.1074/jbc.M609458200
- Primary Citation of Related Structures:
2DRE - PubMed Abstract:
A water-soluble chlorophyll-binding protein (WSCP) is the single known instance of a putative chlorophyll (Chl) carrier in green plants. Recently the photoprotective function of WSCP has been demonstrated by EPR measurements; the light-induced singlet-oxygen formation of Chl in the WSCP tetramer is about four times lower than that of unbound Chl. This paper describes the crystal structure of the WSCP-Chl complex purified from leaves of Lepidium virginicum (Virginia pepperweed) to clarify the mechanism of its photoprotective function. The WSCP-Chl complex is a homotetramer comprising four protein chains of 180 amino acids and four Chl molecules. At the center of the complex one hydrophobic cavity is formed in which all of the four Chl molecules are tightly packed and isolated from bulk solvent. With reference to the novel Chl-binding mode, we propose that the photoprotection mechanism may be based on the inhibition of physical contact between the Chl molecules and molecular oxygen.
Organizational Affiliation:
Department of Biomolecular Science, Faculty of Science, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan.