Structural and mutational analyses reveal the functional role of active-site Lys-154 and Asp-173 of Salmonella typhimurium AphA protein.
Makde, R.D., Gupta, G.D., Mahajan, S.K., Kumar, V.(2007) Arch Biochem Biophys 464: 70-79
- PubMed: 17570338
- DOI: https://doi.org/10.1016/j.abb.2007.03.043
- Primary Citation of Related Structures:
1Z5G, 1Z88, 2AUT - PubMed Abstract:
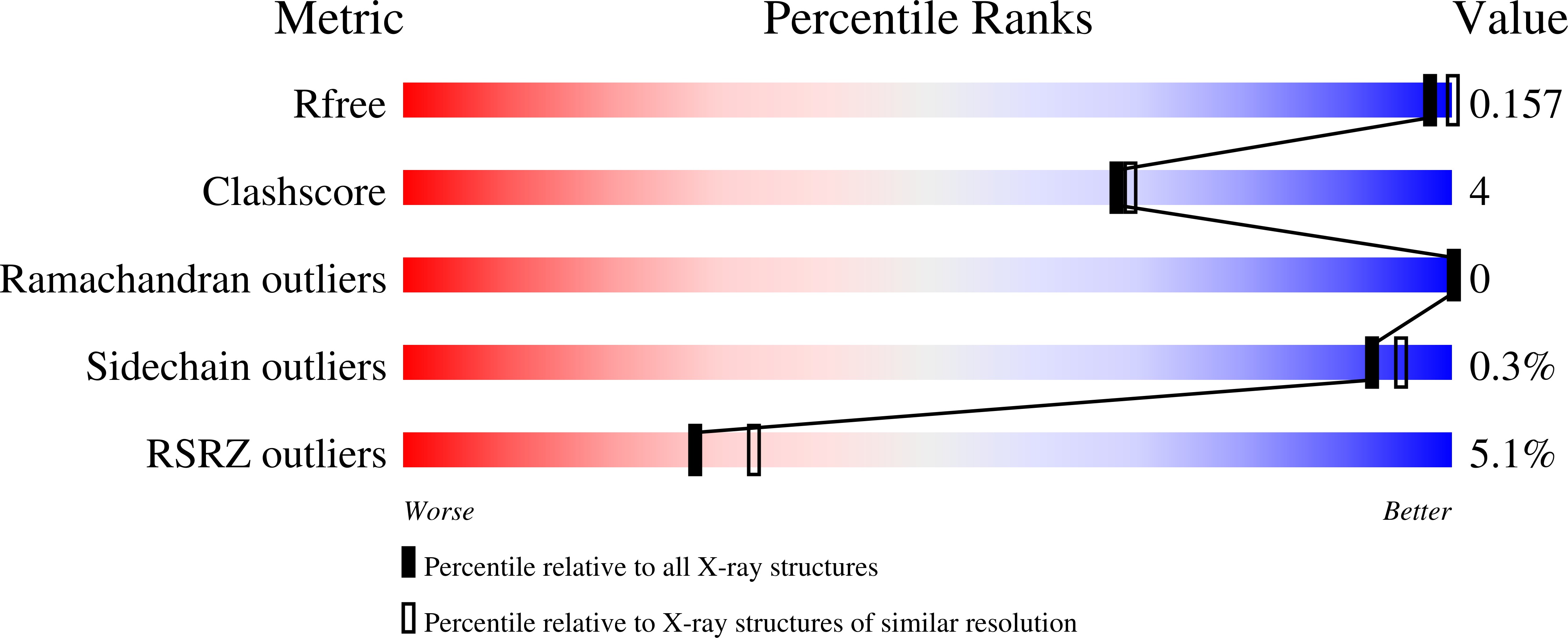
The Salmonella typhimurium class B nonspecific acid phosphatase (AphA protein) belongs to the L2-haloacid dehalogenase superfamily. The conserved Lys-154 interacts with substrate phosphate, nucleophile Asp-46, and Asp-173 in the wild-type AphA protein. Asp-173 also interacts with Mg(II) water ligand and with main-chain amide of loop-4. We report here the mutational analysis of Lys-154 and Asp-173, the crystal structures of the K154N and K154R mutants, and the results of electrostatic potential calculations. The K154N, K154R and D173N mutants display significant reduction in the phosphatase activity. Lys-154 may not be responsible for a juxtaposition of the substrate phosphate and the aspartyl nucleophile, but has an hitherto unknown functional role of rendering the substrate phosphorous atom electron deficient. Nearly 10,000-fold increase in the K(d) value for dissociation of the cofactor Mg(II) observed for the D173N mutant correlates well with theoretically estimated change in the binding free energy of Mg(II).
Organizational Affiliation:
High Pressure Physics Division, Bhabha Atomic Research Centre, Mumbai 400 085, India.