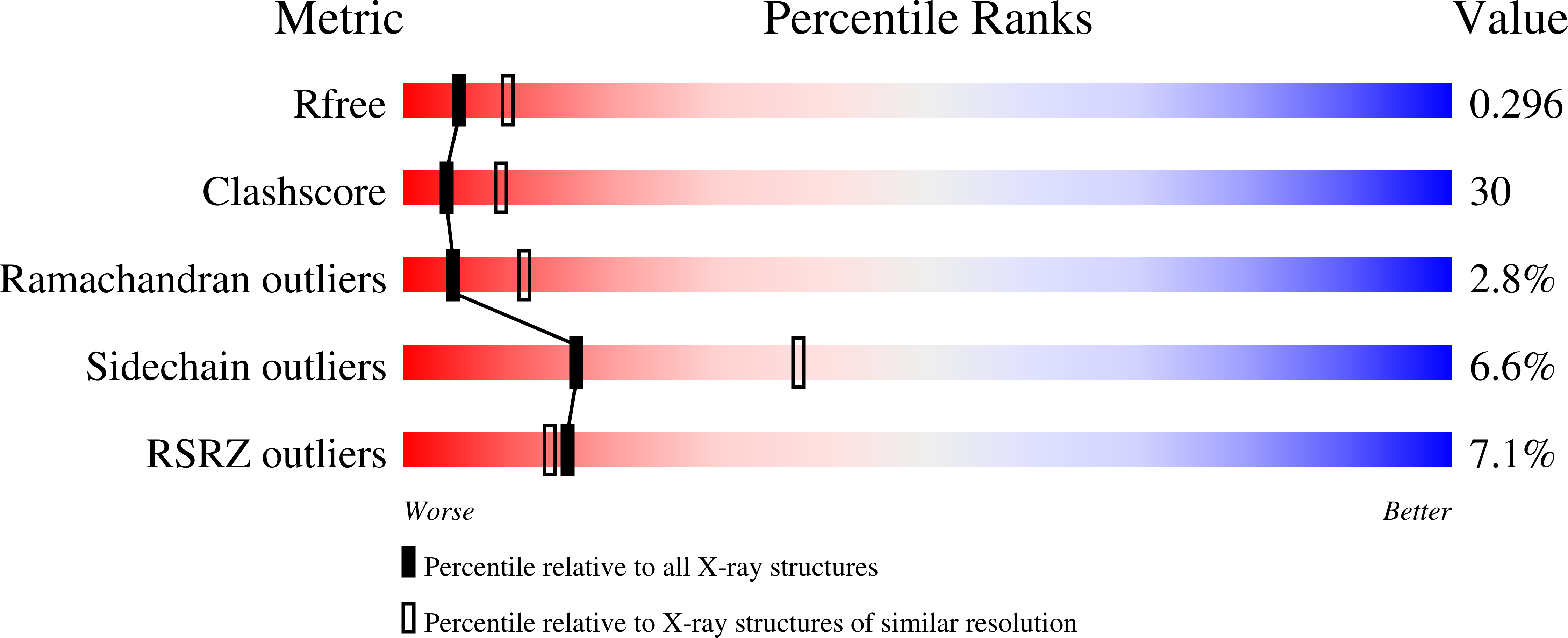
Crystal Structures of the Tricorn Interacting Factor F3 from Thermoplasma acidophilum, a Zinc Aminopeptidase in Three Different Conformations
Kyrieleis, O.J.P., Goettig, P., Kiefersauer, R., Huber, R., Brandstetter, H.(2005) J Mol Biol 394: 787-800
- PubMed: 15893768
- DOI: https://doi.org/10.1016/j.jmb.2005.03.070
- Primary Citation of Related Structures:
1Z1W, 1Z5H - PubMed Abstract:
The tricorn interacting factor F3 is an 89 kDa zinc aminopeptidase from the archaeon Thermoplasma acidophilum. Together with the tricorn interacting factors F1 and F2, F3 degrades the tricorn protease products and thus completes the proteasomal degradation pathway by generating free amino acids. Here, we present the crystal structures of F3 in three different conformations at 2.3 A resolution. The zinc aminopeptidase is composed of four domains: an N-terminal saddle-like beta-structure domain; a thermolysin-like catalytic domain; a small barrel-like beta-structure domain; and an alpha-helical C-terminal domain, the latter forming a deep cavity at the active site. Three crystal forms provide snapshots of the molecular dynamics of F3 where the C-terminal domain can adapt to form an open, an intermediate and a nearly closed cavity, respectively. With the conserved Zn(2+)-binding motifs HEXXH and NEXFA as well as the N-terminal substrate-anchoring glutamate residues, F3 together with the leukotriene A4 hydrolase, represents a novel gluzincin subfamily of aminoproteases. We discuss the functional implications of these structures with respect to the underlying catalytic mechanism, substrate recognition and processing, and possible component interactions.
Organizational Affiliation:
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, D-82152 Martinsried, Germany. kyrielei@biochem.mpg.de