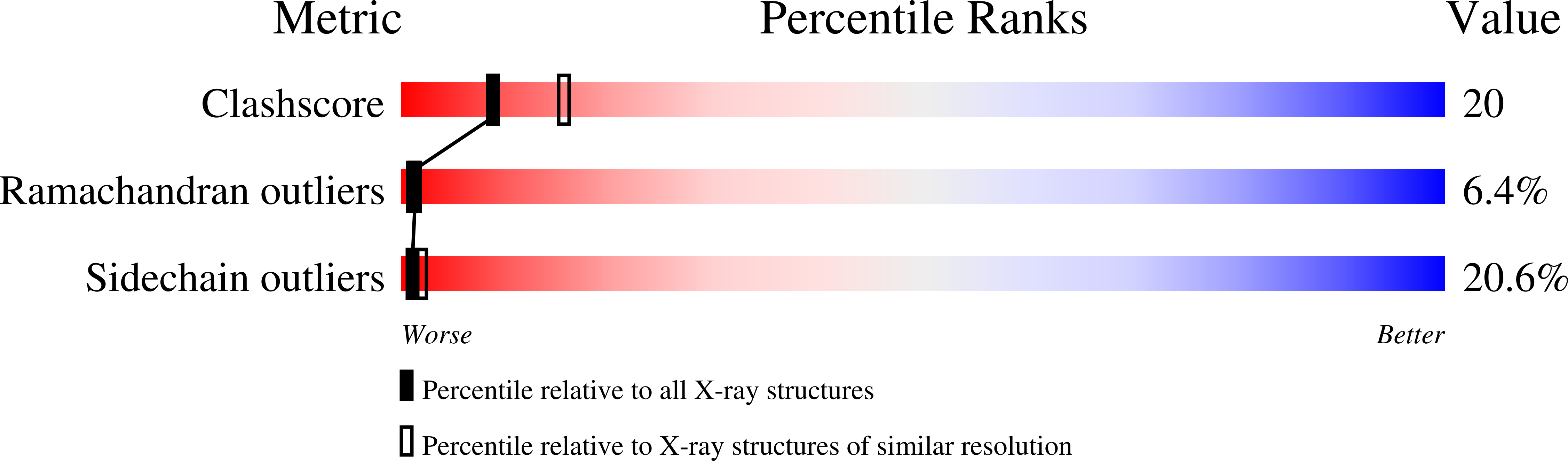The separate effects of E60Q in Lactobacillus casei thymidylate synthase delineate between mechanisms for formation of intermediates in catalysis.
Birdsall, D.L., Huang, W., Santi, D.V., Stroud, R.M., Finer-Moore, J.(1998) Protein Eng 11: 171-183
- PubMed: 9613841
- DOI: https://doi.org/10.1093/protein/11.3.171
- Primary Citation of Related Structures:
1VZA, 1VZB, 1VZC, 1VZD, 1VZE - PubMed Abstract:
X-Ray crystal structures of Lactobacillus casei thymidylate synthase (TS) mutant complexes of E60D with dUMP, and E60Q with dUMP or FdUMP, as well as ternary complexes with folate analog inhibitor CB3717, are described. The structures we report address the decrease in rate of formation of ternary complexes in the E60 mutants. Structures of ternary complexes of L.casei TS mimic ligand-bound TS just prior to covalent bond formation between ligands and protein. Ternary complex structures of L.casei TS E60Q show the ligands are not optimally aligned for making the necessary covalent bonds. Since CB3717 is an analog of the open, activated form of the cofactor, these structures suggest that the slow rate of ternary complex formation in E60 mutants is at least partly the result of impaired alignment of ligands in the active site after binding and activation of the cofactor. Binary complexes of TS E60Q and TS E60D with substrate (dUMP) show no change in dUMP position or occupancy. These results are consistent with the fact that Kd(dUMP) and Km(dUMP) are almost the same, and the rates of folate-independent debromination of 5-bromo-dUMP are even higher than for wild type TS.
Organizational Affiliation:
Department of Biochemistry and Biophysics, University of California, San Francisco 94143, USA.