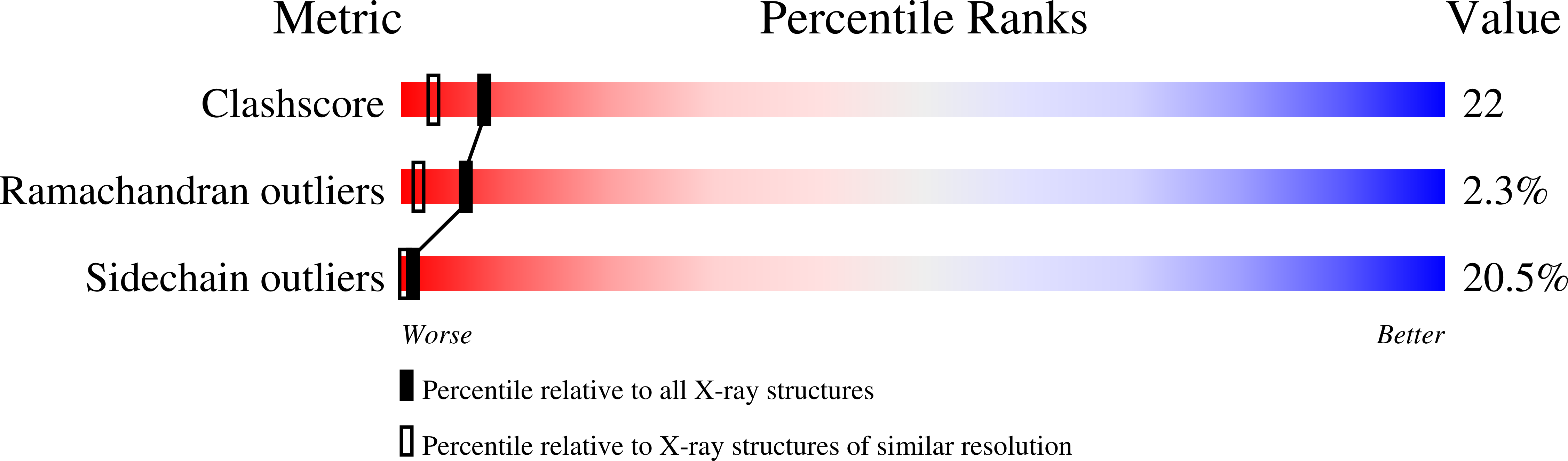Crystal structure of calf spleen purine nucleoside phosphorylase in a complex with hypoxanthine at 2.15 A resolution.
Koellner, G., Luic, M., Shugar, D., Saenger, W., Bzowska, A.(1997) J Mol Biol 265: 202-216
- PubMed: 9020983
- DOI: https://doi.org/10.1006/jmbi.1996.0730
- Primary Citation of Related Structures:
1VFN - PubMed Abstract:
Trimeric calf spleen purine nucleoside phosphorylase has been complexed with hypoxanthine via phosphorolysis of inosine in the presence of phosphate. The resulting, "Michaelis" complex (three hypoxanthine molecules per trimer), presumed to be formed under these conditions, crystallized in the cubic space group P2(1)3, with unit cell dimension a = 94.11 A and one monomer in the asymmetric crystal unit; the biologically active trimer is located on the crystallographic 3-fold axis. High-resolution X-ray diffraction data were collected using synchrotron radiation (EMBL outstation, Hamburg, c/o DESY). The crystal structure has been determined by molecular replacement and refined at 2.15 A resolution to an R-value of 0.18. In the hypoxanthine binding site, a cis-peptide bond between Asn243 and Lys244 is observed. Side-chains of GIu201 and Asn243, as well as one integral water molecule located in the base binding site, form hydrogen bonds with the hypoxanthine N-1 H, N-7 H and O-6. A second water molecule links the base positions N-3 and N-9 with an adjacent pocket, which presumably is the phosphate-binding site. This pocket is filled completely by a cluster of six water molecules. Hence all possible donor/acceptor-positions of hypoxanthine are saturated by hydrogen-bonding to protein side-chains or integral water molecules. Purine nucleoside phosphorylase isolated form human tissues is a primary target for chemotherapeutic intervention, and the more stable calf enzyme has similar physico-chemical and kinetic properties, as well as response to inhibitors. Hence the high-resolution structure presented here may serve for design of inhibitors with potential pharmacological applications.
Organizational Affiliation:
Institut für Kristallographie Freie Universität Berlin, Germany.