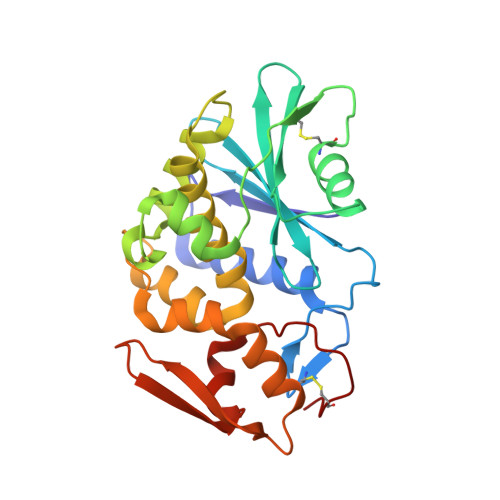
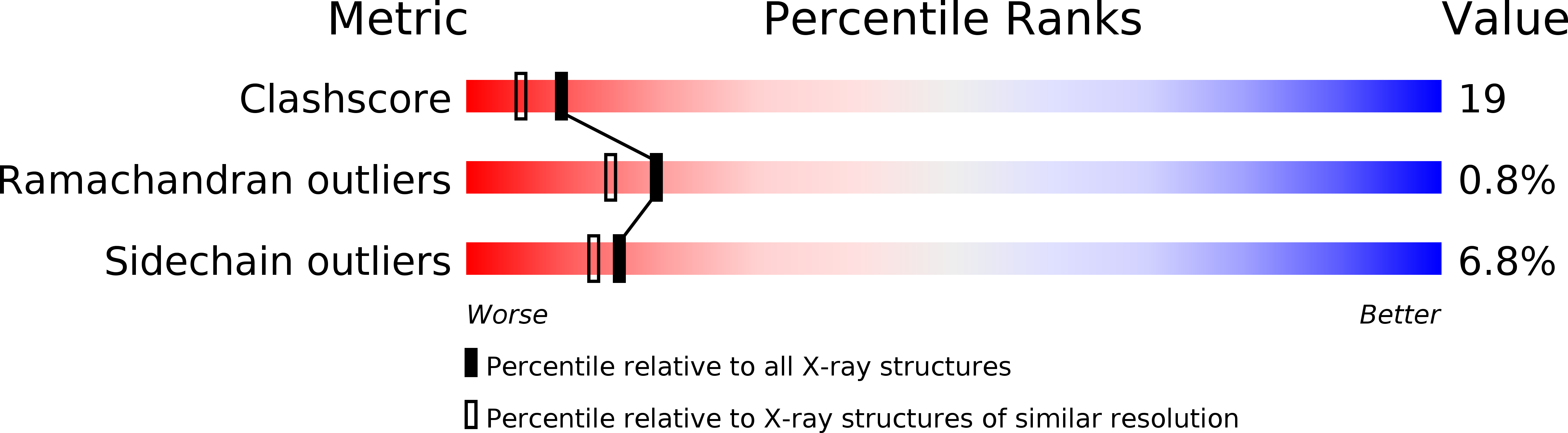X-ray crystallographic analysis of the structural basis for the interactions of pokeweed antiviral protein with its active site inhibitor and ribosomal RNA substrate analogs.
Kurinov, I.V., Myers, D.E., Irvin, J.D., Uckun, F.M.(1999) Protein Sci 8: 1765-1772
- PubMed: 10493577
- DOI: https://doi.org/10.1110/ps.8.9.1765
- Primary Citation of Related Structures:
1QCG, 1QCI, 1QCJ - PubMed Abstract:
The pokeweed antiviral protein (PAP) belongs to a family of ribosome-inactivating proteins (RIP), which depurinate ribosomal RNA through their site-specific N-glycosidase activity. We report low temperature, three-dimensional structures of PAP co-crystallized with adenyl-guanosine (ApG) and adenyl-cytosine-cytosine (ApCpC). Crystal structures of 2.0-2.1 A resolution revealed that both ApG or ApCpC nucleotides are cleaved by PAP, leaving only the adenine base clearly visible in the active site pocket of PAP. ApCpC does not resemble any known natural substrate for any ribosome-inactivating proteins and its cleavage by PAP provides unprecedented evidence for a broad spectrum N-glycosidase activity of PAP toward adenine-containing single stranded RNA. We also report the analysis of a 2.1 A crystal structure of PAP complexed with the RIP inhibitor pteoric acid. The pterin ring is strongly bound in the active site, forming four hydrogen bonds with active site residues and one hydrogen bond with the coordinated water molecule. The second 180 degrees rotation conformation of pterin ring can form only three hydrogen bonds in the active site and is less energetically favorable. The benzoate moiety is parallel to the protein surface of PAP and forms only one hydrogen bond with the guanido group of Arg135.
Organizational Affiliation:
Hughes Institute, Roseville, Minnesota 55113, USA.