Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection.
Shen, Y., Zhukovskaya, N.L., Zimmer, M.I., Soelaiman, S., Bergson, P., Wang, C.R., Gibbs, C.S., Tang, W.J.(2004) Proc Natl Acad Sci U S A 101: 3242-3247
- PubMed: 14978283
- DOI: https://doi.org/10.1073/pnas.0306552101
- Primary Citation of Related Structures:
1PK0 - PubMed Abstract:
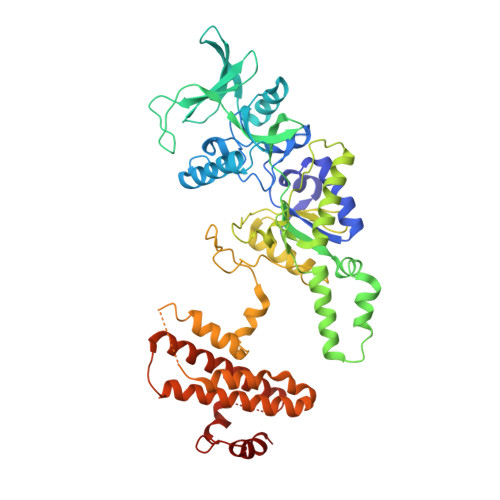
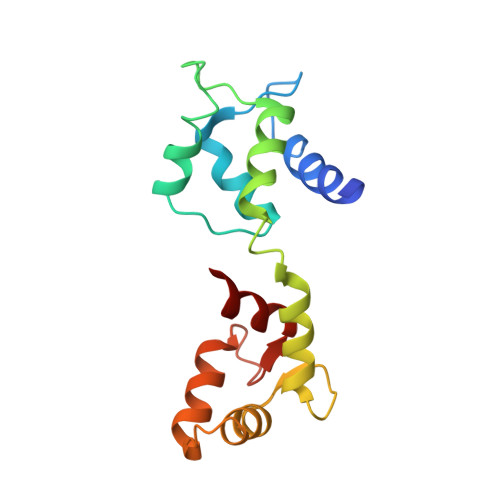
Edema factor (EF), a key virulence factor in anthrax pathogenesis, has calmodulin (CaM)-activated adenylyl cyclase activity. We have found that adefovir dipivoxil, a drug approved to treat chronic infection of hepatitis B virus, effectively inhibits EF-induced cAMP accumulation and changes in cytokine production in mouse primary macrophages. Adefovir diphosphate (PMEApp), the active cellular metabolite of adefovir dipivoxil, inhibits the adenylyl cyclase activity of EF in vitro with high affinity (K(i) = 27 nM). A crystal structure of EF-CaM-PMEApp reveals that the catalytic site of EF forms better van der Waals contacts and more hydrogen bonds with PMEApp than with its endogenous substrate, ATP, providing an explanation for the approximately 10,000-fold higher affinity EF-CaM has for PMEApp versus ATP. Adefovir dipivoxil is a clinically approved drug that can block the action of an anthrax toxin. It can be used to address the role of EF in anthrax pathogenesis.
Organizational Affiliation:
Ben-May Institute for Cancer Research, University of Chicago, 924 East 57th Street, Chicago, IL 60637, USA.