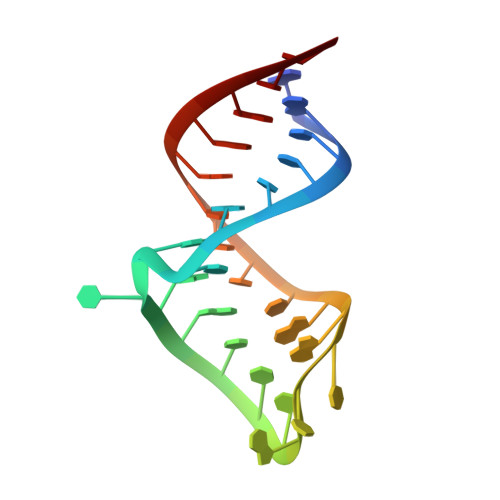
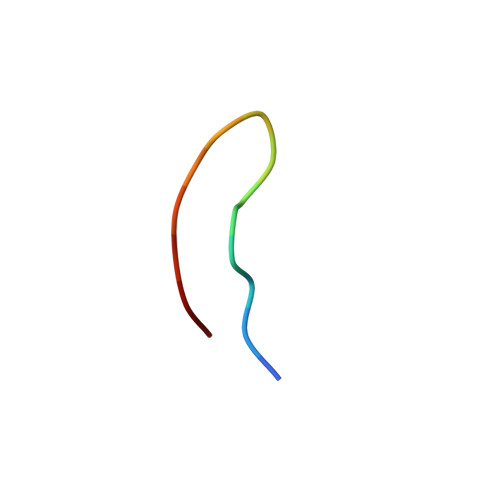Solution structure of a bovine immunodeficiency virus Tat-TAR peptide-RNA complex.
Puglisi, J.D., Chen, L., Blanchard, S., Frankel, A.D.(1995) Science 270: 1200-1203
- PubMed: 7502045
- DOI: https://doi.org/10.1126/science.270.5239.1200
- Primary Citation of Related Structures:
1MNB - PubMed Abstract:
The Tat protein of bovine immunodeficiency virus (BIV) binds to its target RNA, TAR, and activates transcription. A 14-amino acid arginine-rich peptide corresponding to the RNA-binding domain of BIV Tat binds specifically to BIV TAR, and biochemical and in vivo experiments have identified the amino acids and nucleotides required for binding. The solution structure of the RNA-peptide complex has now been determined by nuclear magnetic resonance spectroscopy. TAR forms a virtually continuous A-form helix with two unstacked bulged nucleotides. The peptide adopts a beta-turn conformation and sits in the major groove of the RNA. Specific contacts are apparent between critical amino acids in the peptide and bases and phosphates in the RNA. The structure is consistent with all biochemical data and demonstrates ways in which proteins can recognize the major groove of RNA.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California, Santa Cruz 95064, USA.