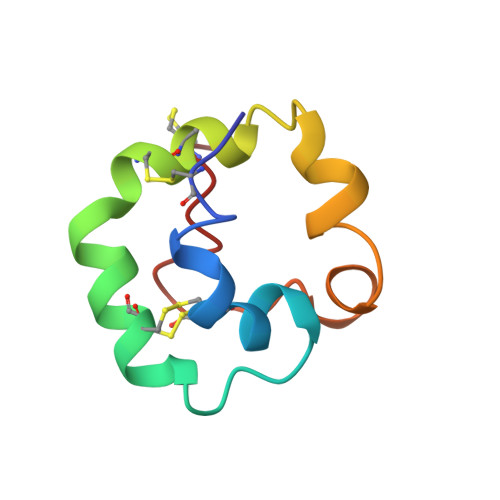
Solution structure of plant nonspecific lipid transfer protein-2 from rice (Oryza sativa).
Samuel, D., Liu, Y.J., Cheng, C.S., Lyu, P.C.(2002) J Biol Chem 277: 35267-35273
- PubMed: 12011089
- DOI: https://doi.org/10.1074/jbc.M203113200
- Primary Citation of Related Structures:
1L6H - PubMed Abstract:
The three-dimensional structure of rice nonspecific lipid transfer protein (nsLTP2) has been solved for the first time. The structure of nsLTP2 was obtained using 813 distance constraints, 30 hydrogen bond constraints, and 19 dihedral angle constraints. Fifteen of the 50 random simulated annealing structures satisfied all of the constraints and possessed good nonbonded contacts. The novel three-dimensional fold of rice nsLTP2 contains a triangular hydrophobic cavity formed by three prominent helices. The four disulfide bonds required for stabilization of the nsLTP2 structure show a different pattern of cysteine pairing compared with nsLTP1. The C terminus of the protein is very flexible and forms a cap over the hydrophobic cavity. Molecular modeling studies suggested that the hydrophobic cavity could accommodate large molecules with rigid structures, such as sterols. The positively charged residues on the molecular surface of nsLTP2 are structurally similar to other plant defense proteins.
Organizational Affiliation:
Department of Life Sciences, National Tsing Hua University, Taiwan 300, China.