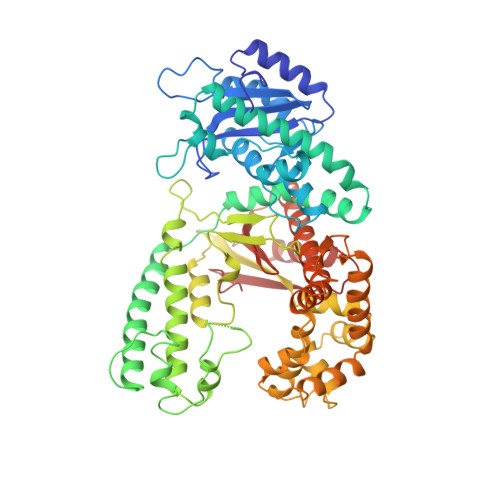
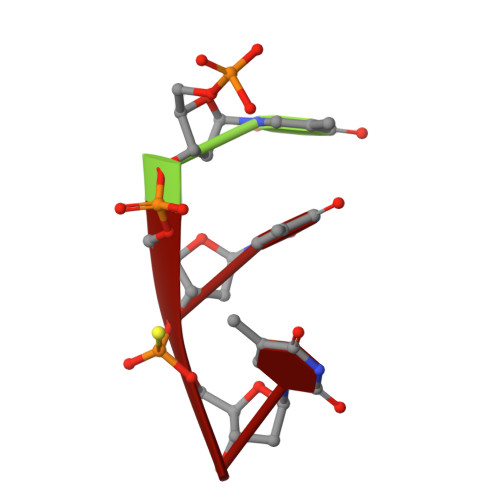
Structural principles for the inhibition of the 3'-5' exonuclease activity of Escherichia coli DNA polymerase I by phosphorothioates.
Brautigam, C.A., Steitz, T.A.(1998) J Mol Biol 277: 363-377
- PubMed: 9514742
- DOI: https://doi.org/10.1006/jmbi.1997.1586
- Primary Citation of Related Structures:
1KFS, 1KRP, 1KSP - PubMed Abstract:
A two-metal-ion catalytic mechanism has previously been proposed for several phosphoryl-transfer enzymes. In order to extend the structural basis of this mechanism, crystal structures of three single-stranded DNA substrates bound to the 3'-5' exonucleolytic active site of the large fragment of DNA polymerase I from Escherichia coli have been elucidated. The first is a 2.1 A resolution structure of a Michaelis complex between the large fragment (or Klenow fragment, KF) and a single-stranded DNA substrate, stabilized by low pH and flash-freezing. The positions and identities of the catalytic metal ions, a Zn2+ at site A and a Mg2+ at site B, have been clearly established. The structural and kinetic consequences of sulfur substitutions in the scissile phosphate have been explored. A complex with the Rp isomer of phosphorothioate DNA, refined at 2.2 A resolution, shows Zn2+ bound to both metal sites and a mispositioning of the substrate and attacking nucleophile. The complex with the Sp phosphorothioate at 2. 3 A resolution reveals that metal ions do not bind in the active site, having been displaced by a bulky sulfur atom. Steady-state kinetic experiments show that catalyzed hydrolysis of the Rp isomer was reduced only about 15-fold, while no enzyme activity could be detected with the Sp phosphorothioate, consistent with the structural observations. Furthermore, Mn2+ could not rescue the activity of the exonuclease on the Sp phosphorothioate. Taken together, these studies confirm and extend the proposed two-metal-ion exonuclease mechanism and provide a structural context to explain the effects of sulfur substitutions on this and other phosphoryl-transfer enzymes. These experiments also suggest that the possibility of metal-ion exclusion be taken into account when interpreting the results of Mn2+ rescue experiments.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520-8114, USA.