Solution structure and stability of the anti-sigma factor AsiA: implications for novel functions.
Urbauer, J.L., Simeonov, M.F., Urbauer, R.J., Adelman, K., Gilmore, J.M., Brody, E.N.(2002) Proc Natl Acad Sci U S A 99: 1831-1835
- PubMed: 11830637
- DOI: https://doi.org/10.1073/pnas.032464699
- Primary Citation of Related Structures:
1JR5 - PubMed Abstract:
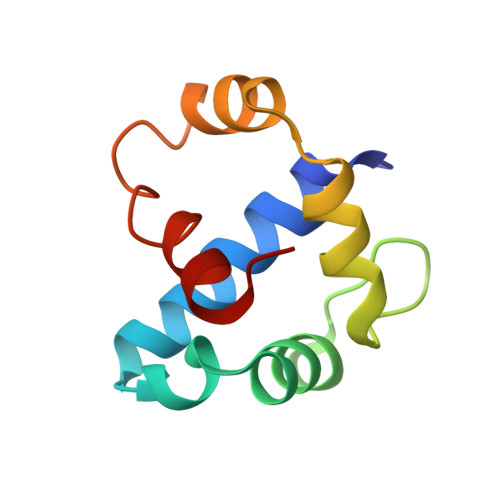
Anti-sigma factors regulate prokaryotic gene expression through interactions with specific sigma factors. The bacteriophage T4 anti-sigma factor AsiA is a molecular switch that both inhibits transcription from bacterial promoters and phage early promoters and promotes transcription at phage middle promoters through its interaction with the primary sigma factor of Escherichia coli, sigma(70). AsiA is an all-helical, symmetric dimer in solution. The solution structure of the AsiA dimer reveals a novel helical fold for the protomer. Furthermore, the AsiA protomer, surprisingly, contains a helix-turn-helix DNA binding motif, predicting a potential new role for AsiA. The AsiA dimer interface includes a substantial hydrophobic component, and results of hydrogen/deuterium exchange studies suggest that the dimer interface is the most stable region of the AsiA dimer. In addition, the residues that form the dimer interface are those that are involved in binding to sigma(70). The results promote a model whereby the AsiA dimer maintains the active hydrophobic surfaces and delivers them to sigma(70), where an AsiA protomer is displaced from the dimer via the interaction of sigma(70) with the same residues in AsiA that constitute the dimer interface.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, KS 66045, USA. jurbauer@ku.edu